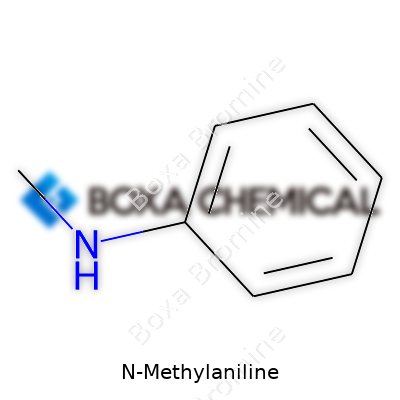
N-Methylaniline: An Industry Mainstay Facing Today’s Challenges
Historical Development
N-Methylaniline first caught the attention of chemists in the nineteenth century, during the early industrial boom that fed countless dye and chemical factories across Europe and North America. In those days, a growing curiosity pushed researchers to examine molecules derived from aniline. After aniline, chemists added a methyl group to the nitrogen, making N-Methylaniline and stumbling onto a compound with sharp potential in both industry and research. Decades of experimentation through World Wars and shifting economies saw this molecule used in emerging fuels, rubber processing, and dye intermediates. The market in the mid-twentieth century valued it not only for its versatility, but also for its ability to tune the properties of bigger, complex chemicals. Like many aromatic amines, N-Methylaniline rode the waves of changing technology, paving the way for today’s dyes, agriculture chemicals, and specialty polymers.
Product Overview
N-Methylaniline presents as a clear, oily liquid with a faint, sharp amine odor. This amine falls under the aromatic amine category and counts as a crucial intermediate for chemicals that impact many everyday applications—fuel additives, pesticides, dyes, and pharmaceutical ingredients all trace roots back to this molecule. Commercial forms range from high-purity grades for pharmaceutical syntheses to more rugged formulations meant for pigments and industrial solvents. Distributors offer variations with tight specifications, taking care to meet both purity and contaminant standards demanded by sensitive electronic and medical fields. Local markets in Asia and the West still drive a steady trade, with pricing and supply always tying back to crude oil, aniline cost, and regulatory pressures on aromatic amine emissions.
Physical & Chemical Properties
Measured by eye, N-Methylaniline comes off as a colorless-to-yellowish transparent oil. Technicians put its boiling point at around 195°C and it tends to freeze near -57°C. Water doesn’t combine well with N-Methylaniline, but alcohols and organic solvents easily dissolve it. A distinct, heavy amine scent signals the molecule’s volatile nature, and it shows enough reactivity with acids and oxidizers to require storage away from strong reactants. On paper, the formula reads C7H9N. The extra methyl group increases its boiling point compared to aniline and boosts solubility in hydrocarbons, making blending with fuels and organic solvents a dependable process. Handling at scale, warehouse staff watch for its high flash point and moderate vapor pressure to prevent mishaps.
Technical Specifications & Labeling
Drums and tanks arriving at facilities list N-Methylaniline’s purity, water content, color scale, and index of refraction. Labs check for trace amounts of aniline, o-toluidine, and other methylated derivatives. For pharmaceutical and electronic uses, buyers demand levels of heavy metals and color less than one part per million, requiring extra purification. Tracking down mislabeling is a real problem, so suppliers print standardized codes, hazard indicators (flammable, corrosive, toxic), and United Nations shipping numbers on every invoice and package. Today’s regulatory standards call for strict batch identification, source tracking, and transparent certificates of analysis from start to finish.
Preparation Method
Industrial plants produce N-Methylaniline by methylating aniline, usually reacting it with methanol in the presence of acid or supported catalysts. Researchers have pushed for greener and more selective methods, such as direct methylation using dimethyl carbonate or C1 feedstocks, but industry mostly sticks to trusted legacy routes: methylate, separate by fractional distillation, and wash away impurities. Older routes using methyl chloride and formaldehyde come with environmental headaches, demanding extra cleanup and tight pollution control. Overall plant efficiency comes down to careful control of temperature and choice of catalysts.
Chemical Reactions & Modifications
Chemists reach for N-Methylaniline when building dye intermediates, pesticides, and custom polymers. Nitration, sulfonation, and acylation reactions take advantage of its activated benzene ring. Reductive amination and further alkylation decorate the molecule for specialty drugs and rubber chemicals. Its lone electron pair on nitrogen encourages formation of Schiff bases, leading to specialty resins and adhesives. During catalytic hydrogenation, N-Methylaniline converts to complex saturated amines. These diverse reactions put it at the heart of both classic and modern organic chemistry synthesis labs.
Synonyms & Product Names
N-Methylaniline has built up a catalog of names: NMA, monomethylaniline, methylaniline, N-methylbenzenamine, and benzenamine, N-methyl-. Commercial blends sometimes show up under manufacturer-specific codes. Any researcher or purchasing manager working with this chemical soon learns to cross-check these synonyms on supplier listings and safety datasheets, since confusion between NMA and aniline causes real safety hazards.
Safety & Operational Standards
On the safety front, N-Methylaniline brings definite risks. Industry guidelines put it under “toxic and harmful” amines, where skin and eye contact cause burns, and inhalation leads to methemoglobinemia—a blood disorder that threatens oxygen transport. Regulations require wearing gloves, goggles, face shields, and using proper ventilation or even fume hoods. Storage rooms use spill containment and intermediates go through rigorous batch sampling for worker safety. Hazmat guidelines from OSHA, the European Chemicals Agency, and the United Nations lay out limits on air exposure, disposal methods, and spill response protocols. Safety training drills and incident logs rank as routine at any facility making or handling this amine.
Application Area
N-Methylaniline has deep roots in the dye, pigment, and rubber industries. For decades, dye makers counted on this amine to give vivid blues, greens, and ultra-stable blacks for textiles and inks. Pesticide and herbicide formula designers tap its aromatic backbone for reliable pest control agents. Octane-boosting fuel additive recipes blend NMA to improve gasoline performance and meet environmental standards, though health and pollution concerns have led to tighter rules. Drug researchers use it to build up active ingredients in the lab. Polymer chemists like myself have reached for NMA when customizing resins and engineering plastics to new specifications.
Research & Development
Recent research circles around greener synthesis and biodegradation studies. Universities and corporate labs have chased new catalysts that limit side products and cut out toxic reagent waste. Environmental chemists look for better ways to trap or convert NMA in waste streams. Advances in analytical chemistry let researchers trace residues in groundwater and air, helping local governments understand exposure risks. Machine learning models now predict toxicity and reactivity better, pointing to a future where new, safer analogs replace the original compound in sensitive applications. Drug designers use NMA as a starting scaffold and map new derivatives for both improved pharmacological action and lower toxicity.
Toxicity Research
N-Methylaniline does not get a clean bill of health from toxicologists. Studies show that skin, inhalation, or digestive exposure can lead to the formation of methemoglobin, which starves body organs of oxygen. Chronic, low-level exposure causes anemia and damage to liver and kidneys. Early studies connected the molecule with cancer risk in industrial workers, especially those without regular monitoring or protection. Animal studies led to regulatory limits on workplace air concentrations set by OSHA and their European counterparts. Many industrial biologists and safety experts now hunt for ways to knock down emissions and capture spills before NMA poisons the local watershed.
Future Prospects
N-Methylaniline faces both opportunity and pressure in the future. As environmental regulations grow stricter, producers lean into cleaner synthesis and closed-loop recycling to stay in business. Downstream industries look for alternatives for fuel additives and colorants, pushing R&D dollars toward safer aromatic amines. Some companies have started using NMA as a model system for green chemistry education, training the next wave of synthetic chemists. Digitalization lets plants monitor leaks and exposures with sensors and AI-informed alarms. If regulation gaps close and the world shifts toward even tighter chemical controls, N-Methylaniline’s future will hinge on smarter risk management, better waste destruction tech, and novel uses in areas economists don’t see coming today.
Understanding N-Methylaniline’s Place in Everyday Products
N-Methylaniline sounds like something tucked away in a chemistry lab, but it weaves through many parts of life you’d never expect. Many folks might not recognize it by name, yet it's a key piece in the puzzle of what runs quietly behind industries, especially energy and chemical manufacturing.
Boosting Gasoline: Why Refiners Reach for N-Methylaniline
At the gas pump, we all expect smooth engine performance—nobody enjoys a sputtering ride or frustrating stops. N-Methylaniline has a big part to play here since it helps raise the octane rating in gasoline. Higher octane improves how fuel burns, so engines run more efficiently and with fewer knocking issues. That means better reliability and, often, more loyal customers for fuel brands. The compound doesn’t just ‘sit’ in the mix; it actively interacts with combustion chemistry, making gasoline more robust under higher compression in engines.
From Dyes to Pharmaceuticals: Its Hidden Reach
This isn’t just about drivers and gasoline companies. N-Methylaniline also works as a stepping stone for producing certain dyes. Textile companies depend on color consistency. Having stable, vibrant colors gives clothing brands a real edge and keeps customers coming back. Before synthetic dyes, color didn’t last. N-Methylaniline helped change that. It allows stronger chemical bonds in dye products, keeping colors attached to fabrics through sweat, washing, and sunlight.
Pharmaceutical companies keep finding new angles on molecules like this. Some medicines rely on methylation—the process that takes substances like aniline and turns them into more useful forms. Research shows N-Methylaniline acts as a building block for making drugs, letting manufacturers tweak formulas in ways that help them create longer-lasting or more potent medicine. Safety is huge here—no shortcuts, since small missteps can mean big risks for patient health.
Working with N-Methylaniline: Risks and Realities
Hazards stick close to this chemical. Unprotected exposure can cause irritation or even deeper health issues if folks working in manufacturing plants aren’t careful. That’s why the industry leans hard on personal protection, good ventilation, and strict rules about how to handle spills or leaks. Factories audit their safety processes often and invest in better monitoring equipment, putting people’s well-being as a higher priority. It’s a decent reminder that profit shouldn’t outweigh safe practices. Learning from mistakes keeps the doors open and communities healthy.
Finding a Path Toward Responsible Use
As clean energy pushes forward, some experts ask tough questions about long-term reliance on compounds like N-Methylaniline. The world keeps moving toward transparent, sustainable solutions. Improvement can show up in small steps: tighter storage guidelines, cleaner disposal methods, and sharing best practices across borders. Research into green chemistry sometimes leads to smart substitutes, cutting down on risky byproducts without losing the benefits these chemicals bring. Connecting companies with researchers, opening conversations with workers, and listening to communities shape a safer future for everyone using or living near these products.
Clear information and open dialogue build trust. Making N-Methylaniline safer and more sustainable doesn’t just help industries—it helps everyone, from workers in overalls to shoppers picking up brightly colored t-shirts. That’s worth talking about.
N-Methylaniline: Not Just Another Chemical
N-Methylaniline pops up in research labs and a few niche industries. I once saw a friend of mine, a chemical engineer, work with it while developing specialty dyes. He shared something that stuck with me: respect for risks matters, not just knowledge. Anyone who’s ever caught a whiff of this stuff knows it’s no cakewalk. That sharp, amine smell isn’t just annoying—it’s the sign you should only be handling it with a plan in place.
Direct Contact: Risks You Can’t Ignore
N-Methylaniline loves to sneak through skin and right into your bloodstream. When handling, skin exposure can set off symptoms like headaches, dizziness, or worse, methemoglobinemia—which steals oxygen from your blood. You might not even notice right away. Eyes sting and water if hit with a splash. I saw someone wipe sweat from their forehead in a rush and learned the hard way: gloves and goggles aren’t optional. Industrial-grade gloves (like nitrile ones) block it better than the thin stuff from the grocery store.
The Air You Breathe: Ventilation Makes a Difference
Many chemical injuries happen not from splashes, but from vapors. N-Methylaniline evaporates easily, creating an invisible problem. You can’t “see” what you’re breathing in. Fume hoods, local exhaust, and keeping containers sealed—every simple habit matters. If you’re working in a small shop or home lab, invest in real ventilation, not just an open window. Cheap paper masks won’t cut it against vapor. Air-purifying respirators with organic vapor cartridges cut down risk, but require fit testing and proper use.
Clothing: What Works in Real Life
Clothes aren’t created equal in the lab. I’ve watched people just throw on a cotton lab coat and think they’re covered. Over time, liquid can seep in. Chemical aprons, splash goggles, closed-toe shoes—these basics have saved more than a few people from long-term problems. Remove contaminated clothing right away. Don’t assume detergent will handle it; set up dedicated laundry options if possible to keep chemical traces away from everyone else in the house or facility.
Spills and Cleanup: Response, Not Panic
Spilling N-Methylaniline kicks off instant stress. Don’t grab paper towels or sweep it up with a broom. Clear the area, ventilate, and use absorbent materials designed for organic solvents. Dedicated spill kits—including absorbent pads and proper disposal bags—make all the difference. After my friend’s lab had a spill, they trained everyone, not just the techs, in cleanup. That meant no one scrambled or made mistakes. Collect the material and label waste properly for hazardous collection. Flushing it down the drain causes bigger headaches, especially for local wastewater systems.
Storage: More Than Just Locking the Door
N-Methylaniline beats up on standard plastics and reacts with strong acids. Flame-proof, ventilated cabinets keep the grime off containers, but also lower fire risk. Labels must be big and clear, not scribbled shorthand. Inventories and regular checks flag any leaks before things escalate. Keep it away from food, and never reuse chemical containers for drinks or snacks—a mistake I’ve seen lead to real danger.
Training: The Hidden Key
People respect what they understand. Regular safety training, walk-throughs, and drills catch gaps. Bring in outside experts for harsh chemicals like this—sometimes, that fresh pair of eyes spots what locals miss. I’ve picked up tips from experienced colleagues whose stories drove the lesson home in ways rulebooks never could. Reviews of the latest guidelines from the National Institute for Occupational Safety and Health (NIOSH) help keep everyone up to speed and confident.
Practical Solutions
Treating N-Methylaniline with seriousness starts with personal protective equipment and ends with the right knowledge. Small changes—using proper gloves, maintaining ventilation, training every hand—help make sure nobody pays the cost of overlooking safety. I’ll never forget the stories I’ve heard or the lessons they taught. Chemicals always deserve our respect, and it’s those routines and habits that make all the difference at the end of the day.
What the Formula Tells Us
N-Methylaniline has the chemical formula C7H9N. This simple line of letters and numbers packs in a lot — it tells you the whole story about how many carbon, hydrogen, and nitrogen atoms work together in a molecule that matters in both industry and research.
I remember my first look under the hood of a benzene ring with a nitrogen atom attached, and realizing just how transformational a small group, like a methyl (–CH3), can be. Here we see an aniline skeleton: a benzene ring bonded to an amine group. Swap one hydrogen from that amine with a methyl group, you get N-Methylaniline. Simple change, totally different properties.
Peering at the Structure
Think of the structure as a benzene ring — six carbons locked in a flat hexagon, each bonded to a hydrogen. Attached to one of these carbons, a nitrogen delivers that essential amine character. Instead of being linked to two hydrogens, the nitrogen in N-Methylaniline cradles a single hydrogen and one methyl group. If you draw it, that methyl group dangles off the nitrogen, never directly touching the benzene ring.
This kind of structure means you get electron-donating effects from both the methyl and the nitrogen. People who work with organic chemicals know how much of a difference that makes — shifts in boiling point, solubility, and reactivity. That’s one reason the textile and pharmaceutical worlds keep it handy.
Why Structure Matters in Everyday Life
In my lab, I saw more than one instance where substituting a methyl group for a hydrogen changed the story with contaminants and solvents. The extra carbon and three hydrogens make N-Methylaniline an oilier substance compared to its parent aniline. It dissolves well in most organic solvents, less so in water. In manufacturing, that bit of hydrophobicity helps N-Methylaniline serve as an intermediate in dyes, stabilizers, and lubricants.
Health and safety come up quickly. The methyl group increases lipophilicity — meaning N-Methylaniline can slip more easily past biological membranes. That can cause trouble, since higher tissue uptake often follows. Data says high exposure connects with blood disorders due to methemoglobinemia, so engineering controls and careful handling take priority.
Learning from Its Role in Industry
Industry leans on compounds like N-Methylaniline for efficiency and cost savings. For those mixing fuel additives, its high octane-boosting properties attract attention. Companies aiming to improve the efficiency of gasoline used this molecule for years, although environmental concerns and stricter regulation have clipped its use.
Wastewater coming out of chemical plants sometimes holds traces of this compound — I’ve seen firsthand how water treatment teams work with activated carbon and advanced oxidation to clean it up. It’s not just chemistry, it shapes policy and environmental engineering.
Toward Safer and Cleaner Chemistry
Better science points to more responsible production and management. Teams now track workplace exposure, monitor releases, and push for safer alternatives when possible. Green chemistry approaches have nudged manufacturers to develop new intermediates that don’t carry the same health baggage.
Research, peer review, and transparency help communities understand what’s in their air and water. Real expertise on the ground, shared freely, builds the trust and knowledge to handle molecules like these safely, for everyone’s benefit.
Everyday Precautions in Chemical Handling
N-Methylaniline has a place in several different industries, most notably as a component in fuel additives and dyes. For many people only familiar with gasoline or household cleaners, the reality of handling something like N-Methylaniline can seem pretty distant. In actual workplaces, though, dealing with chemicals like this becomes routine. Routine shouldn't mean careless. One mistake, and there are real consequences: health risks, environmental contamination, or even property damage from fire or leaks.
Hazards that Deserve Respect
N-Methylaniline doesn’t get along with heat, sunlight, or open flames. It’s flammable, so storing it near hot equipment, welding setups, or engines causes real trouble. Even a small spark can set off a fire that puts people and property at risk. There are people who still remember storage room accidents because someone ignored the flammability warnings on a chemical drum. A little oversight can turn costly, fast.
This chemical also evaporates easily. Breathing in its vapors can lead to dizziness, headaches, or much worse with prolonged exposure. It can harm the liver or kidneys, according to research cited by the National Institute for Occupational Safety and Health. Nobody should treat these risks as just technical details in a safety data sheet. Proper ventilation and proper protective gear matter as much as safe driving or good machine maintenance.
Environmental Factors Matter
Good storage doesn’t just mean pushing drums into any empty corner. N-Methylaniline demands containers made from materials that don’t corrode or let vapors leak out. I’ve seen glass bottles used by labs and metal drums lined with special coatings for bulk stocks. Low-tech solutions like secondary spill trays catch leaks, which keeps both workers and the building safe from chemical stains or fumes. For larger stockpiles, chemical warehouses use dedicated rooms with temperature controls. These aren’t luxuries; cool, dark, dry storage stretches the life of every shipment and stops the chemical from breaking down into something more dangerous.
Compliance Isn’t a Checkbox
Keeping up with local and federal regulations isn’t just about avoiding fines. In the United States, OSHA sets out clear guidelines for storing flammable liquids. Fire codes often demand explosion-proof lighting and limits on how much can stay in one spot. Insurance claims for fire damage sometimes fall apart if the right measures aren't in place. These aren't just bureaucratic hurdles—they protect real people.
Simple Steps, Big Impact
Everyday solutions help avoid disaster. Regular inspections of storage containers can catch drips or corrosion before they get worse. Proper labeling, visible from every angle, means whoever handles the chemical knows instantly what’s inside. Staff who get regular safety briefings—and actually listen—handle emergencies better, whether that’s a spill, a fire, or someone complaining about the smell.
In the end, safe storage of N-Methylaniline isn’t rocket science. It’s about keeping flammable chemicals in a cool, well-ventilated, out-of-the-way spot, using the right containers, and giving the risks the full attention they deserve. With a mix of the right tools, honest respect for what the substance can do, and some careful planning, most problems never get the chance to start.
What People Need to Know About N-Methylaniline
N-Methylaniline isn’t a household name for most folks, but it shows up in industries where chemicals play a central role—especially in fuels and some dyes. The risk comes from its properties: a clear, oily liquid with a strong odor that can slip into the body through breathing, skin contact, or swallowing. Workers in fuel blending facilities or those living near chemical plants have a higher chance of exposure, and storing or using N-Methylaniline without safeguards sets off alarm bells.
Health Hazards Linked to N-Methylaniline
After years spent around chemical storage sites, there’s no missing the signs of trouble with compounds like N-Methylaniline. Breathing in fumes causes not just headaches or dizziness but can make you feel like the world is spinning. The real worry starts with long-term exposure. People have ended up with blood trouble—specifically, methemoglobinemia, where blood can’t carry enough oxygen, causing fatigue and blue-tinged skin. If you spend enough time around this chemical, liver and kidney issues also begin to stack up.
Data from the National Institute for Occupational Safety and Health (NIOSH) shows workers who regularly handle this chemical must follow tough safety rules. NIOSH recommends full skin and respiratory protection even at low levels, because repeated exposure multiplies the health risks. For most folks, these dangers feel distant, but once someone brings it home on their skin or clothes, the risk becomes part of daily life.
Environmental Impact of N-Methylaniline
The land and water feel the weight of this chemical, too. Spills and leaks at refineries or storage facilities leave N-Methylaniline soaking into soil and finding its way into waterways. In my experience volunteering with river cleanup crews, even small releases can stress fish and plants. Aquatic creatures seem most vulnerable. They suffer changes in behavior or flat-out die when exposed, even at levels below those that would harm people.
Reports from the Environmental Protection Agency paint a stark picture: the compound doesn’t break down quickly in nature. It builds up, especially if safety checks slip or disposal rules get ignored. Communities relying on well water worry about contamination because just a little can taint a lot. Modern testing catches this early, but not every community has real-time monitoring or resources to tackle a spill.
Possible Solutions and Safer Practices
To lower the risks tied to N-Methylaniline, industries need airtight handling, better training, and emergency plans that everyone can follow. After seeing workers cut corners on personal protective gear, there’s no ignoring the importance of simple things like gloves, goggles, and regular air checks. Plants using this chemical can swap in safer additives or invest in closed systems that keep the chemical out of open air. On the government side, stronger rules about storage, labeling, and waste handling build layers of defense.
Communities near these plants want real information and alerts about any incidents or releases. Investment in cleanup technology makes recovery faster, but prevention always costs less than dealing with an emergency. By pushing for tighter controls and keeping an open line between companies and the people affected, damage to health and the environment drops off. In the end, making N-Methylaniline safer for everyone comes down to vigilance and honest responsibility from those using and regulating it.