N-Bromosuccinimide: Commentary on Its Development, Uses, and Future
Historical Development
The story of N-Bromosuccinimide traces back to the early half of the twentieth century. Back then, scientists needed reliable ways to add bromine to organic molecules without the hassle and unpredictability of elemental bromine. NBS, as folks in the lab call it, stepped in to fill that gap. Its introduction was more than just another chemical launch; NBS gave organic chemists a sturdy tool for selective bromination. Chemists in the 1950s and 1960s, working on natural product synthesis and the first wave of pharmaceutical development, leaned in heavily. The precise control it offered over radical bromination—especially at allylic and benzylic positions—opened up reaction pathways that old-school reagents couldn’t touch without byproducts and mess. That early reputation for reliability set NBS apart, and even in today’s modern labs, it remains a staple on the synthesis shelf.
Product Overview
N-Bromosuccinimide isn’t flashy, but it always gets the job done. It shows up as an off-white crystalline powder with a faint odor, content to sit in a resealable bottle until called into action. Chemists reach for it mainly because it dishes out bromine atoms in a tidy, measured way. Unlike pure bromine—liquid, corrosive, hard to handle—NBS plays well with others and supports a controlled, less hazardous work environment. Over the years, it’s earned hundreds of different catalog numbers and product names across chemical supply companies, but they all boil down to the same practical, dependable reagent for radical reactions and mild brominations.
Physical & Chemical Properties
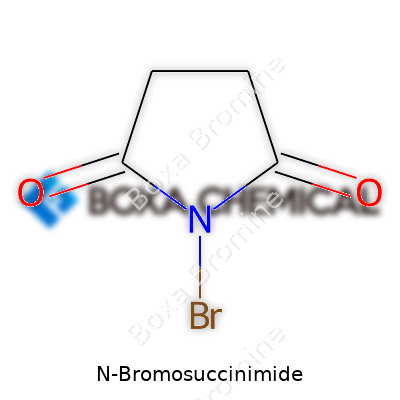
Glancing at its stats, N-Bromosuccinimide has a molecular formula of C4H4BrNO2 and a molar mass a touch over 177 g/mol. It usually turns up as colorless or faint yellow crystals that start to decompose at around 173°C—nothing dangerous unless you try heating it with disregard. The crystals don’t exactly melt so much as they break down, which means NBS doesn’t easily evaporate or sublimate, and stays workable in a laboratory setting. Its solubility sits on the modest side; it isn’t going to dissolve into every solvent you throw at it, but blends into dimethylformamide, dioxane, and water with gentle heating. Holding a moderate oxidizing character, NBS deals out bromine at a more manageable pace than sodium bromate or elemental bromine.
Technical Specifications & Labeling
Buyers and lab techs don’t just want a bottle labeled “NBS”—they want details. Regulatory bodies mandate a CAS number (128-08-5), signal words for hazards, and information about purity, often over 98%. Suppliers stamp hazard symbols for skin, eye, and respiratory irritation, along with recommendations to keep it cool, dry, and sealed up. The safety data sheet often runs several pages, but chemists usually skim for melting point, storage tips, and shelf life. For those scaling up reactions, manufacturers offer bulk packaging, certificate of analysis, and batch traceability, now standard for anything going into medical or food precursors. Laboratories expect a lot from a simple salt, and NBS consistently makes the grade.
Preparation Method
Small-scale synthesis of N-Bromosuccinimide in the lab rarely runs into complications. The go-to route still involves dripping bromine into a cold, slushy concoction of succinimide and aqueous sodium hydroxide. Temperature control matters—keep things cool and slow to avoid runaway reactions or decomposition. After separating the solid and giving it a few washes, filtering, and drying, chemists get a pretty pure NBS ready for most synthetic plans. On the industrial side, the same principles apply, though automation and better control systems replaced the old beakers and ice baths. The simplicity and efficiency of this synthesis helped NBS win its place in bulk catalogs everywhere, a case where the classic approach steers clear of unnecessary complexity.
Chemical Reactions & Modifications
Anyone who has worked in an organic chemistry lab knows there’s a real bounty of reactions where NBS leads the show. At the top sits allylic and benzylic bromination, a reaction so routine it gets taught in every undergraduate lab guide. Its mild radical conditions leave sensitive functional groups untouched while swapping in a bromine atom right where you want it. This predictable reactivity comes from the steady release of bromine in situ, usually sparked off by light or a radical initiator like AIBN. NBS also pitches in for selective oxidation reactions, converting alcohols to aldehydes under specific conditions. In peptide chemistry, it breaks down tryptophan and other side chains with precision, helping map protein structure. Over time, researchers have tinkered with the base molecule, altering the succinimide ring or swapping in other halogens, but the original still dominates. Chemists always appreciate a tool they can trust.
Synonyms & Product Names
Open a catalog from any chemical supplier and you’ll spot NBS hiding under a laundry list of names: N-Bromosuccinimide, 1-Bromopyrrolidine-2,5-dione, and succinbromimide top the charts. Synonyms help bridge language and regional differences, keeping supply chains flowing smoothly. As new synthesis companies entered the fold, branded versions with proprietary grades and purity claims hit the shelves. Despite the jumble of catalog names, the chemical identity has never wavered, a rare consistency in a marketplace full of additives and rebranded chemicals.
Safety & Operational Standards
No matter how familiar chemists get, safety never takes a back seat with NBS. Its oxidative strength can irritate eyes, skin, and lungs, so working under a fume hood is non-negotiable. Careless contact or spilled powder raises risks of burns and breathing problems. Containers need to stay tightly closed, away from acids and bases that could trigger an unwanted reaction. Labs keep calcium chloride in desiccators nearby, since even a little moisture can start breaking NBS down and reduce effectiveness. Regular training and drills matter, especially since NBS often gets used in undergraduate labs where inexperience creates risks. While accidents stay rare, every lab keeps protocols fresh, PPE at hand, and safety data sheets on standby.
Application Area
The list of applications might surprise anyone outside chemistry circles. Besides being a go-to for selective bromination, NBS plays a quiet but crucial role in making pharmaceuticals, agricultural chemicals, and specialty dyes. Pharmaceutical companies lean on it when synthesizing active molecules with tricky bromine substitutions—an example straight from real-world drug development. Industrial labs rely on its clean reactions and track record for scaling up to pilot batches. Outside pure synthesis, NBS helps in analytical chemistry, labeling biomolecules and mapping out metabolic pathways in medical research. Creative synthetic chemists keep finding new reactions, sometimes using NBS with microwaves, ultrasound, or green solvents to improve yields, cut waste, and support sustainability goals.
Research & Development
R&D teams continue to test the boundaries of what NBS can do safely and efficiently. In the last decade, one focus has been adapting NBS reactions to greener or solvent-free techniques—real progress for chemists seeking to shrink the environmental footprint of synthesis. Another avenue explores using NBS as a starting point for more complex reagents, attaching groups that let it target unusual positions in organic molecules. It makes sense to look for ways to keep the best features—selectivity, predictability—while smoothing out drawbacks like waste bromine and timing issues. Tech companies now design miniaturized, automated platforms that feed NBS into microreactors, harnessing its power for high-throughput screening. R&D doesn’t just chase bigger yields; it also asks hard questions about safety, cost, and environmental impact, pushing suppliers to improve purity and reduce trace contaminants that can ruin sensitive syntheses downstream.
Toxicity Research
Toxicology teams have taken a comprehensive look at how NBS affects humans and the environment. NBS irritates mucous membranes and the skin, especially in pure form. Chronic exposure or inhalation damages lung tissue and, in severe cases, can lead to pulmonary inflammation—real consequences for workers in bulk production or folks handling large-scale bromination. Ingested by accident, it causes nausea and gastrointestinal issues, and the material can burn if it gets into cuts or eyes. Toxicity to aquatic life is well documented, as brominated byproducts persist in water and soil. Regulators have responded, listing NBS as a hazardous substance for shipping and storage, with strict reporting for environmental releases. Most working chemists already respect these risks and take proper precautions, but reminders matter. Updates to labeling and production practices continue to improve workplace safety and environmental resilience, a testament to the ongoing collaboration between researchers, industry, and regulators.
Future Prospects
Looking ahead, NBS appears set to keep playing a foundational role in organic synthesis. The chemical industry faces mounting pressure to cut environmental impact, so expect more research into recycling spent reagents and developing biodegradable analogs. Process chemists push for greener, safer alternatives, but also stress the need for reagents with NBS’s unique selectivity. Newer areas like flow chemistry and microreactor design rely on predictable, homogeneous reagents—another fit for NBS as labs upgrade away from batch processes. Innovations in packaging, such as single-use capsules and coated granules, reduce handling risks for both industry veterans and undergraduates just learning the ropes. As regulations tighten and waste streams face closer scrutiny, companies work on recovery methods for brominated byproducts, reclaiming value and protecting the environment along the way. The tried-and-true features of NBS—reliability, versatility, ease of use—give it a firm spot in research and industry. The challenge is evolving without losing what gave it staying power in the first place, and meeting new standards for safety, efficiency, and sustainability.
Understanding N-Bromosuccinimide's Main Role
N-Bromosuccinimide, better known as NBS among chemists, holds a special place on the laboratory shelf. In synthetic organic labs, people reach for NBS when they need to brominate a compound with precision, often in places other methods leave untouched. My first serious encounter with NBS was during a late-night session tackling allylic bromination for a senior thesis. The results spoke for themselves: clean reactions and minimal byproducts. This isn’t just a student win; it reflects why so many chemists trust NBS to add bromine atoms right where they want them, especially in complex molecules.
Why NBS Is More Than Just a Reagent
The appeal of NBS goes beyond its performance in the bottle. A lot of halogenation reactions can get out of control, giving rise to mixtures you never intended. With NBS, people in the lab notice a difference—the reactions often move forward in a controlled manner. That repeats in published research, too. Scientifically, NBS generates low, steady concentrations of bromine radicals, meaning it avoids dumping too much reactive bromine at once. This slows chaotic side reactions, making clean-up much easier.
Year after year, NBS keeps showing up in protocols beyond the basics. In the pharmaceutical world, it helps carve out specific “handles” on molecules for further transformation. This step finds value when working on new drugs or agrochemicals. Small-scale producers and process chemists still pull NBS off the shelf for this reason. It has earned trust because it gets the job done without requiring fancy or dangerous setups.
Real-World Benefits and Concerns
NBS isn’t magic, though. Every chemical in the lab comes with trade-offs. There’s the safety angle: while NBS feels more predictable than elemental bromine or harsh liquid bromine reagents, it’s still an irritant, and the succinimide byproduct can complicate waste disposal. My own experience with an unexpected spill proved that nothing is truly “benign” when bromine is involved. Gloves, careful handling, and a fume hood are not optional.
In education, professors often include NBS in advanced organic labs to show students a cleaner path through bromination. This hands-on experience demonstrates how one reagent can help avoid messy purifications. Textbooks mention NBS alongside an ever-growing list of new reagents, but it still earns its chapters for good reason. The chemistry community passes down knowledge about the quirks and uses of NBS through generations.
Where NBS Fits Into Future Development
Environmental impact and green chemistry goals add pressure to rethink halogenation. NBS sidesteps some of the problems linked to traditional bromination approaches, yet it still relies on bromine chemistry, which isn’t always kind to living systems or waste streams. I remember a group at my old department tinkering with alternatives based on NBS’s framework, working to swap out bromine for less troublesome atoms. Their work underscores a real point: yesterday’s solutions start today’s conversations about safer practices.
Even as researchers push for change, NBS keeps finding value. It serves its purpose where control, selectivity, and accessibility matter. That’s worth remembering, both for what it brings to the table and for the questions it leaves open for the next generation of chemists.
Why People Take N-Bromosuccinimide Seriously
N-Bromosuccinimide, or NBS, finds a spot on the lab bench for a reason. It gets the job done on selective brominations and oxidations, but those very properties make it hazardous. You can probably remember a time in a lab when carelessness with a powdered reagent led to a coughing fit or a chemical burn. The risks feel real once you’ve been there.
Breathe Carefully: Inhalation Risks Matter
NBS releases fumes that catch unprepared lungs off guard. Even a little exposure can irritate the airways. In one summer research gig, I watched a grad student sniff an unmarked vial—he regretted it instantly, coughing his way out of the hood. The lesson stuck: wearing a mask and always working inside a functioning fume hood isn't about following a checklist; it's about keeping your basic health in check. The hood pulls those fumes away from your face. Respirators meant for organic vapors add backup if you can't guarantee good airflow.
The Gloves Rule: Avoiding Contact
NBS burns skin, plain and simple. Your choice of gloves affects safety more than you'd think. Nitrile stands up well, whereas thin, cheap latex barely lasts a few spills. Years ago, I ignored a ripped nitrile glove for "just one more transfer"—a mistake that ended with a red burn on my palm. NBS stains and burns sneak up quickly. Eye protection with side shields means you won’t find yourself rushing to an eyewash station because of one careless splash.
Storage and Cleanup: Not the Place for Shortcuts
Storing NBS in a sealed, dry container blocks out moisture and light that break it down into nasty byproducts. Don’t stick NBS next to acids, bases, or fuels—those mixtures lead to instability and unpredictable reactions. Cleanup might seem tedious after a long session, but those white stains sitting on your spatula become a real source of danger. Double-bag disposable items and toss them into the designated chemical waste. Tools that touched NBS deserve a thorough cleaning; a simple rinse and dry only spreads risk around the lab.
Spill Response: Practice Pays Off
Spills almost always happen right when you least want them. One time, a sloppy transfer caused a small NBS mound on a benchtop. Rather than panic, the teaching assistant grabbed a scoop, swept it gently, and used damp paper to mop up the traces. They followed with a 10% sodium thiosulfate rinse. The whole group learned quickly from that real moment: spill kits need to stay stocked and ready—no excuses. If a spill occurs, limit it, protect yourself, and follow established neutralization steps. Wash your skin with plenty of water if contact happens. Replace contaminated clothing before it spreads further.
Training and Team Culture
Safety doesn’t start and end with warning signs or checklists. It’s the mindset you build, sharing reminders with new students, staying alert, and never treating repeat procedures as routine. One overlooked task can bring real harm. If your team skips training or cuts corners, risk multiplies. So, set an example, review incident reports, and always talk about near-misses. The more people know, the less likely anyone walks away hurt.
Why Storage Matters More Than People Realize
N-Bromosuccinimide shows up a lot in labs, especially for making organic molecules or controlling certain chemical reactions. Folks who spend time with chemicals know that some are much fussier than others. N-Bromosuccinimide is one of those chemicals that can quickly cause a problem if left unattended or stored without care. You leave it out, and moisture in the air will react with it, changing its character and even making it risky to handle. Stories of ruined batches or even accidents can be traced back to someone just placing the bottle on the wrong shelf.
Humidity and Sunlight: Enemies of Stability
Anyone who has worked in a student or industrial lab has probably run into old bottles of N-Bromosuccinimide caked at the top, sometimes even crusted inside. That’s what happens if you let too much water or light get to it. The chemical reacts and starts to lose strength, and worse, it generates bromine gas, which you definitely do not want floating around. Direct sunlight speeds up this breakdown. Sealing up the lid tightly and keeping the container out of light should be automatic, almost like the habit of double-checking the gas tap.
Treat It Like a Treasure—Keep It Cool and Sealed
Any chemist who cares about their work puts this compound away after use, preferably in a tightly sealed glass container. The plastic ones that are common in some places might not offer the same barrier, so glass is safer. Set the container in a cool spot; lab refrigerators work well, but even a dedicated storage cabinet set below room temperature prevents unwanted reactions. Researchers I’ve worked with always slipped their NBS bottle inside a zip bag or stored it alongside desiccant packs. Nobody wants their starting material damp or discolored.
Safe Storage, Safe Hands
N-Bromosuccinimide doesn’t just lose its punch when stored badly—it becomes more hazardous. Laboratory safety data make it clear: exposure to fumes of degrading NBS can cause skin irritation, and breathing those fumes is worse. Decades of lab safety protocols stress the point because chemical accidents often sneak up when folks cut corners with storage. Good storage habits protect not just the current team but anyone who comes across the shelf tomorrow.
How to Make It Routine
Make simple changes and it pays off. Marking dedicated containers and keeping an updated log avoids confusion and lets everyone know what’s inside and how old it is. Some labs even assign somebody the task of checking and rotating chemical stock each month, just to catch bottles that need replacing before trouble starts. Installing moisture indicators next to sensitive chemicals gives another layer of warning if something is going wrong. It’s not about expensive equipment—just being consistent and smart about where things live.
Taking Responsibility for Chemical Care
I have watched a brilliant reaction flop because someone grabbed a bottle of NBS that looked fine but had been sitting in sunlight on a windowsill. Just one lapse like that wastes hours of work and could endanger people. The answer isn’t paranoia, just respect for what the substance can do and a steady habit of following steps that experience and chemical safety data guides have already laid out. A labeled, sealed bottle stored in a cool, dry, dark spot might not seem glamorous, but it keeps reactions running and people healthy.
Understanding N-Bromosuccinimide
N-Bromosuccinimide, often called NBS, carries more weight in synthetic chemistry than people often realize. Wherever one looks in advanced organic labs, a jar of this compound sits alongside reagents considered essential for selective reactions. Folk who’ve spent time working through aromatic substitutions or radical brominations see NBS not as some strange formula but a reliable partner in tough transformations.
Chemical Structure and Formula
A quick look at a sample of NBS tells part of the story: white crystalline powder, a hint of its strong oxidative nature escaping even at room temperature. Chemically, NBS carries the molecular formula C4H4BrNO2. This simple formula translates to a structure where a succinimide ring—a five-membered ring with two carbonyl groups at positions 2 and 5—attaches to a bromine atom on the nitrogen at position 1.
Visualizing it brings back plenty of time spent scribbling on notepads. Picture this: start with a simple succinimide backbone, keeping two carbonyls lined up across from each other. Slap a bromine atom on the ring’s nitrogen, and you’ve got NBS. It doesn’t get much more efficient than that. This structure makes it a reliable source of low, steady bromine concentrations when mixed into a reaction—an invaluable property that no clunky test tube ever delivers alone.
Why NBS Structure Matters in Chemistry
Some folks never stop to question why NBS matters structurally until a yield drops or selectivity vanishes. NBS offers cleaner reactions than elemental bromine, which acts far more unrestrained and dangerous to both people and products. The two carbonyls on the ring draw electron density away from the nitrogen, making the attached bromine easier to release without wild side reactions. NBS doesn’t flood a reaction with an uncontrolled halogen; it doses radicals or electrophiles bit by bit.
This property means more predictable outcomes in allylic brominations. Think about chemistry as a busy workshop. Messy tools lead to errors, wasted resources, and safety incidents. NBS works more like a precision screwdriver instead of a hammer, giving synthetic chemists a tidier approach to introducing bromine into molecules.
Practical Impact and Solutions to Challenges
In research, inconsistency strikes hardest when reagents decompose. I've handled NBS that clumped from exposure, spoiling reactions and wasting time. Keeping NBS dry and cool, stored away from sunlight and moisture, tackles much of this. Suppliers today ship it in sturdy, light-blocking glass, and experienced chemists insist on using only fresh product for sensitive syntheses.
NBS does not solve every bromination challenge, though. Strong bases or acids break down its structure faster than intended, reminding folks it demands respect and careful pH control. Anyone with a robust ventilation hood and gloves can avoid most trouble, but clean results arrive with good storage habits and proper reaction setup, never pure luck.
Building Stronger Knowledge
Bringing this knowledge together matters far beyond the lab. Pharmaceutical development, advanced plastics, and even agrochemical research use NBS as a trusted step in broader syntheses. Recognizing its chemical structure and formula helps demystify why so many industries reach for it, and why newcomers in chemistry labs everywhere quickly learn to respect and rely on NBS’s unique capabilities.
Everyday Handling in the Lab
Bottles of N-Bromosuccinimide (NBS) tend to show up tucked into supply cabinets in most synthetic chemistry labs. Over the years, I’ve cracked open dozens myself—noisy lids, a waft of something chlorine-like, crystalline powder inside. Chemists rely on consistency in this white chemical, because a lot rides on the way this compound behaves during bromination reactions. One bad bottle wastes time and reagents, messes up yields, and adds to frustration. The first check always centers on purity. Most commercial sources ship NBS above 98%, with some reaching 99%. If you spot a value below that, think twice before running a sensitive reaction—stray contaminants can shut down selectivity, boost side products, or spark safety problems.
What Purity Really Means
This purity number isn’t just marketing. Manufacturers use protocols like iodometric titration or HPLC to confirm the active bromine content. Reliable suppliers add a certificate of analysis to each batch, usually specifying appearance (white to off-white crystals or powder), melting point (173-176°C), and loss on drying (less than 0.5%, so it won’t clump from trace water). These are not just trivial lines in a spec sheet. Soft, brownish NBS or one that melts way below range screams degradation. In my experience, those “bargain” sources online with vague paperwork often come with issues—either polymeric junk that stops reactions cold or enough insoluble gunk to clog filtration setups. Years of missed deadlines and failed scale-ups have taught many chemists to demand a clear, well-documented bill of health for every lot.
Safety Tied to Specification
Many people overlook the safety angle. Impure NBS can form explosive byproducts under certain conditions. Flaky quality control caused more than a few high school and undergrad incidents that could have ended badly. Imagine a small batch failing to brominate, only to find piles of tarry residue that heat up out of nowhere. Having a predictable, high-quality product reduces these risks. The spec sheet’s attention to hazardous contaminants—like heavy metals, organic impurities, or leftover solvents—matters far beyond academic nitpicking. Laboratories that keep safety top of mind often partner only with suppliers who have strong track records and openness around their QC processes.
Quality Control from Start to Finish
It’s easy to skim part numbers and place an order, but lot-to-lot consistency calls for deeper diligence. I’ve seen research halted by subtle differences in purity—one shipment triggers a fat, clean NMR peak, the next turns reactions into a guessing game. Some vendors offer pharmaceutical or ACS reagent grades, which cost more but lower headaches. Many university labs run incoming samples through their own quick purity checks: test reactions or a simple melting point. If results seem off, a call to the supplier sorts things out, and repeat problems usually get the axe from the approved vendors list.
Keeping Commercial NBS Reliable
In my work, the best solution to purity headaches combines three habits: ask for and review the certificate of analysis every time; keep NBS cool, dry, and away from sunlight; and push suppliers about their manufacturing transparency. Buying larger lots sometimes ensures uniformity but brings shelf-life risks, so splitting stock or cooperatively testing across research groups keeps bad batches out of circulation. High-quality NBS is one of those basic requirements—overlooking it causes more trouble than anyone has patience for.