Hexachloroethane: In-Depth Analysis and Practical Insights
Historical Development
Hexachloroethane came out of the early 20th-century push for new substances to feed the demands of a fast-modernizing world. Chemists noticed the compound’s usefulness during wartime, using it in smoke munitions and signaling devices. Military stockpiles drove production for decades, especially from the 1930s to the 1970s. By the late 20th century, tighter environmental rules started forcing careful evaluation of how Hexachloroethane moved through industries, with research highlighting risks tied to exposure and persistence in soils and groundwater. Engineers and scientists wrestled with balancing economic interests against the rising tide of health concerns. The historical arc shows a familiar pattern: discovery leads to enthusiasm, demand grows, and regulation eventually changes the landscape.
Product Overview
Hexachloroethane sits in a chemical family known for heavy chlorination and a tendency to stick around in the environment. The pure material forms a colorless crystalline solid at room temperature. It shows up in stores as tablets, granules, or blocks, depending on end use. The compound drew attention not just for its performance but also for how it pairs well with additives in specific formulations. In industries like metallurgy, its application in removing oxygen from molten metals helped raise product quality. Regular folks likely encountered it more indirectly—through products or processes where chemical purity and controlled reactions matter.
Physical & Chemical Properties
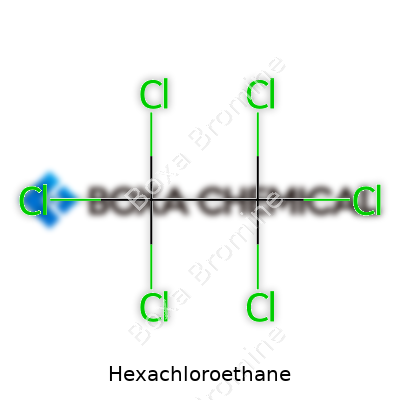
Hexachloroethane (C2Cl6) weighs in with a molar mass near 236 grams per mole. Its melting point hovers around 187 °C and it boils at roughly 185 °C under reduced pressure, decomposing before reaching atmospheric boiling point. The compound resists dissolution in water but dissolves well in organic solvents like chloroform and benzene. As one might expect from a chlorinated hydrocarbon, it acts as a nonpolar molecule with a slow rate of degradation in open air and groundwater. I’ve found it takes months for samples to visibly yellow on a lab shelf, which speaks to its chemical stability—a mixed blessing considering environmental persistence.
Technical Specifications & Labeling
Most chemical suppliers offer Hexachloroethane with purity levels above 99%, though trace impurities such as tetrachloroethylene and hexachlorobutadiene sometimes linger. Labels on containers include hazard pictograms (skull and crossbones, exclamation mark), signal words like “Danger,” and statements on organ toxicity and environmental harm. UN number 1185 helps handlers identify it during transport, a precaution born from mishandling incidents in past decades. Requirements for storage remain clear: keep it cool, dry, and away from open flames or incompatible chemicals such as strong bases, reducing agents, and metals like sodium. These rules did not appear out of thin air—they reflect lessons learned after accidental spills, unexpected fires, and health complaints surfacing over years of industrial usage.
Preparation Method
Commercial-scale Hexachloroethane often relies on controlled chlorination of simple hydrocarbons, typically ethylene or tetrachloroethylene, using excess chlorine gas under pressure. In a well-ventilated plant, operators pump ethylene, then add a steady flow of chlorine, using a catalyst at about 80–120°C. A scrubbing system catches byproducts like phosgene and hydrogen chloride. Distillation and recrystallization follow, with careful separation to keep unwanted polychlorinated compounds out of the final batch. Having observed these operations, attention to leak prevention and off-gas capture stands out, since small lapses escalate risk for everyone on-site.
Chemical Reactions & Modifications
Hexachloroethane doesn’t just sit around—it can serve as a chlorinating or reducing agent under certain conditions. When burned with metals like aluminum, it creates dense smoke from the formation of metal chlorides. This made it a staple in grenades for troop concealment. In the lab, chemists use it for deoxygenation reactions or to introduce chlorine into organic frameworks. At high temperatures or with UV light, it tends to break down, giving off various chlorinated fragments. It rarely reacts explosively, but combined with reactive metals or strong reducing agents, runaway reactions can kick up more toxic byproducts like phosgene. Watching these reactions from the bench, the smell and color changes sound the alarm for even the most experienced hands.
Synonyms & Product Names
The chemical answers to many names: Hexachloroethane, perchloroethane, carbon hexachloride, ethane hexachloride, C2Cl6, and old trade names from chemical catalogs that date back to early industrial chemistry. Every name anchors it to a different application—military specs use one code, while metallurgists prefer another. This spectrum of terms occasionally clouds regulatory discussions or shipping transactions, highlighting the need for clear cataloging and cross-referenced material safety data sheets.
Safety & Operational Standards
Handling Hexachloroethane takes more than gloves and goggles. Its vapor irritates eyes and lungs, especially in enclosed spaces. Chronic exposure links with liver, kidney, and blood disorders, and animal testing tied it to cancer and reproductive effects. Good practice means closed systems, local exhaust ventilation, and strict adherence to OSHA and ACGIH limits—often below 1 ppm in workroom air. In foundries, workers learned the hard way that even short-term smoke from molten metal cleaning impacts breathing for days. Decontamination and personal protection protocols must cover not just the main operator, but anyone downwind or working near waste streams.
Application Area
Hexachloroethane built its reputation in foundry and military circles. Its mix with aluminum powder, for example, generated heavy smoke for signaling or obscuring troop movements. In metal processing, it pulls oxygen from molten metals like aluminum and magnesium, improving alloy quality. The compound plays a smaller role today in pyrotechnics and pesticide production, partly because of environmental restrictions and concerns about dioxin and other byproducts. Waste management and recycling still encounter it in contaminated soils or sludge, which raises the stakes for proper cleanup and chemical tracking. My own work in industrial hygiene circles saw legacy sites wrestling with old stockpiles and equipment coated in strange white residues.
Research & Development
The last couple decades brought a tighter focus on environmental behavior, alternative deoxygenating agents, and less toxic replacements in chemical synthesis. Researchers track breakdown pathways in soil, water, and biological systems, learning that complete destruction proves harder than many assumed. Breakthroughs now often center on remediation: improved filtration, bioremediation approaches, and advanced oxidation methods to tackle stubborn residues. Chemists keep probing smaller, less persistent molecules that could one day replace Hexachloroethane in specialty reactions. Every lab result feeds back into industry standards, as companies seek cost-effective, safer products without losing the performance record built over generations.
Toxicity Research
Toxicity studies trace the journey from animal labs to workplace accidents and environmental monitoring. Inhaled Hexachloroethane affects the central nervous system, causing dizziness, confusion, and—at high levels—loss of consciousness. Animal studies found liver and kidney damage, and the EPA flagged it as a probable human carcinogen. Blood and urine analysis in exposed workers showed accumulation of breakdown products for weeks after a single heavy exposure. Regulators stepped in with tighter limits on air, water, and soil concentrations; meanwhile, medical surveillance programs in some industries now monitor workers for early signs of organ stress. My time consulting with health and safety teams opened my eyes to how subtle symptoms can build until a whole crew needs reassignment.
Future Prospects
Hexachloroethane faces a crossroads. Companies that once relied on its unique chemistry seek alternative routes for the same functions—smoke production, metal refining, or chlorination—without leaving a legacy of persistent pollutants. Investment in cleanup technologies ramps up as new sites get identified and global trade tightens standards for hazardous shipments. As regulations evolve, demand grows for compounds that break down faster and carry fewer health risks. Chemists keep searching for safer stand-ins with the same reliability under industrial conditions, though none hit every target so far. The story reflects a broader shift: weigh short-term utility against long-term health and environmental costs, and adapt practices as new data shapes public understanding and corporate responsibility.
Looking Past the Complex Name
Hexachloroethane doesn’t pop up in most everyday chats, but it starts to sound familiar if you’ve ever worked in metal casting or spent time around a military base. The world doesn’t run on glamorous materials alone—a lot of progress depends on chemicals like this one, with its sharp, camphor-like odor and crystalline grayish-white form.
Real-World Jobs for Hexachloroethane
This compound most famously turns up in smoke grenades and pyrotechnics. Tossing one of those military grenades, soldiers create a thick cloud to cover movement. Hexachloroethane sits at the core of that reaction, burning up in hand-thrown grenades to deliver essential cover. Not many have reason to grab one, but for people on the ground dealing with actual threats, that smoke makes a difference.
On the industrial side, hexachloroethane serves a quieter but crucial purpose in metal foundries. Workers producing brass, bronze, and other copper alloys rely on it as a degassing agent. During melting, hydrogen bubbles sneak into the metal. Drop in a hexachloroethane tablet, and the chemical helps draw those bubbles out. Cleaner metal pours mean fewer defects and stronger finished products—hard to argue with results like that.
Beyond the Factory Floor
Old-school fire extinguishers used hexachloroethane too, but that’s less common now. Some folks still remember the dry chemical units that could break up oil fires or electrical blazes. Veterinary medicine, at one point, turned to the compound in an attempt to treat certain parasites in livestock, but those uses mostly faded as better solutions and stricter safety rules came along.
Concerns and Straight Talk
All chemicals have a cost, and this one brings baggage. Direct exposure harms the liver and kidneys, and early studies in animals point to cancer risks. Workers in foundries or armories—even in the United States—report symptoms ranging from headaches to breathing trouble after a day around those fumes. The Environmental Protection Agency keeps a close watch. In some places, regulators cracked down or banned use in non-essential products, especially since safer alternatives can do some of these jobs.
Living near an industrial plant myself, I’ve seen how production choices ripple out into everyday routine. Air quality dips, and the trade-off between local jobs and clean air comes up at city council meetings, not just in lab reports. Nobody argues that manufacturing should vanish, but it’s fair to ask companies to pick safer chemicals whenever science offers a replacement.
Room for Improvement
Newer degassing agents and safer formulations for smoke products have started to work their way into factories, but switching over tools and training takes time and money. For countries or businesses where cost is everything, change lags behind. Big policy shifts—like government-funded trade-in programs or incentives for cleaner tech—can speed things along.
Talking about chemicals like hexachloroethane gets tricky without painting it as pure villain or retro hero. The jobs it handles are still needed, but future work in metal shops and security training grounds can rely less on legacy substances when practical options open up. Insisting on health and safety, weighing the cost, and trusting data over nostalgia—that’s the only way this conversation moves forward.
What Is Hexachloroethane and Where Does It Show Up?
Hexachloroethane turns up in some strange places. Folks in metalwork use it for welding and cutting, especially in making smoke screens or degassing metals like aluminum. I’ve seen it used sometimes in pyrotechnics, fire suppression, and even as a lab reagent. All that science talk makes it sound distant, but the reality hits close to home because of how it affects workers, animals, and the environment.
The Hazards Add Up Fast
The main warning comes from its toxic nature. The US Environmental Protection Agency includes hexachloroethane on its list of hazardous substances. Breathing it in, getting skin contact, or, worst of all, ingesting it can lead to some real health problems. Inhaling dust or smoke laced with hexachloroethane triggers coughing, chest tightness, or trouble breathing. My neighbor who is a welder once told me about headaches, dizziness, and nausea on days with poor ventilation. He didn’t realize those fumes had long-term effects stacked on top of the immediate misery.
Repeated exposure leads to damaged livers, corroded kidneys, and, in some studies with lab animals, cancer. The National Institute for Occupational Safety and Health marks it as a potential occupational carcinogen. Some research points to reproductive problems. Waterways catch runoff from factories, and fish absorb it. EPA’s Superfund list has spots contaminated by hexachloroethane across the country. Even folks living a few miles from old dump sites get worried about drinking water safety.
Tackling the Risks: What Can Be Done?
Personal protective equipment won’t fix everything on its own. Masks, gloves, and good ventilation only go so far. Companies save money by skimping on safety investments, but that ends up costing everyone more through medical bills and environmental liability. A real solution shines through when businesses use less dangerous substitutes for the same tasks, or design processes to keep exposure close to zero. Awareness matters—training workers to spot trouble and handle spills or fumes keeps accidents from spiraling.
Regulators can help by forcing better reporting of where hexachloroethane gets stored or used. I’ve seen some companies dodge questions about chemical inventories. Strong rules, clear safety labels, and inspections tip the scales toward safer handling. Local activists sometimes push for cleanup at contaminated sites, and honest reporting about what’s in the water or air helps residents make smart choices about their health.
Community Matters More Than You Think
Hexachloroethane poses a serious risk, but people standing up for each other help hold polluters accountable. If workers speak out about unsafe jobs, and neighbors keep asking questions, it gets harder for dangerous chemicals to hide in plain sight. The burden of toxic exposure falls everywhere—from welders in the plant to families near chemical waste dumps. By supporting regulations, demanding transparency, and listening to those closest to the issue, communities can break the pattern of chemical risks getting swept under the rug.
A Look at What’s on the Line
Hexachloroethane isn’t a chemical anyone tosses in a cupboard and forgets about. Over the years in the lab and working with professionals who run warehouses, I’ve seen firsthand the consequences of missing the basics on hazardous substances. Hexachloroethane has been used in smoke grenades, some metal refining, even in old fire extinguishers. It’s been banned for several uses for a reason. Everything about its storage and handling comes down to limiting human and environmental risk.
Getting Storage Right
Start with a cool, well-ventilated spot. Warm climates speed up chemical breakdown; nobody wants volatile vapors creeping into workspaces. Set Hexachloroethane apart from other chemicals, especially anything that could react, like strong acids or bases. Dedicated, corrosion-resistant shelving or containers matter. I’ve watched rust eat through weaker storage bins, risking spills no one wants to clean up. Labels must stay clear, readable, and up to date. If you’ve ever scrambled during a containment drill, you know labels are more than just a box-ticking exercise.
Moisture poses a quiet threat by corroding containers and encouraging leaks. Always check for dry, sealed conditions. Keeping chemicals off the ground helps in flood-prone areas. Keep a spill kit close—absorbent pads, gloves, goggles, and an emergency plan simplify those tense moments when a container ruptures or seeps.
Handling: More Than Avoiding Spills
Every ounce handled deserves respect. Proper gloves, splash-proof goggles, lab coats—never seen anyone regret taking a few extra seconds to gear up. Ventilation matters, too. Fumes from Hexachloroethane don’t always set off alarms, but they carry health risks. Short-term exposure can bring headaches or nausea. Longer-term, the risks deepen, with links to liver and kidney trouble. In my career, I’ve seen workers ignore ventilation, thinking short exposure did no harm—until they paid for it after years on the job.
Bulk moves? Use transfer systems designed for chemicals. No makeshift siphons, no open pouring. Static electricity sounds harmless, but in dry storage it can build up, risking ignition. Grounded equipment reduces the chance of those rare but scary incidents. Never eat, drink, or touch your face when handling chemicals. No exceptions—this isn’t up for debate.
Respecting Regulation—And Health
Hexachloroethane has run into bans and restrictions worldwide. In the U.S., the Environmental Protection Agency and OSHA set exposure limits and rules. Not following rules can lead to stiff penalties, but more important, it jeopardizes health. Following the SDS directions and local guidance keeps workplaces safe and communities from contamination. People trust companies handling chemicals to take the safest route. In my view, that trust ranks higher than a quarterly savings on warehouse overhead.
Room for Improvement
Many sites focus on just storage. They overlook readiness for leaks, employee training, or keeping emergency numbers fresh. Routine drills with your actual crew—and not just the names on compliance forms—lock in safe habits. Investment in staff training brings down accident rates more than any storage equipment can. Leveraging digital inventory and monitoring cuts human error.
Every workplace I’ve known that takes these lessons seriously keeps incidents low and morale high. Skipping simple steps or underestimating Hexachloroethane endangers more than just the immediate team. Responsible storage and handling don’t just meet rules—they protect lives.
The Basics of Hexachloroethane
Hexachloroethane keeps things simple on paper: its chemical formula reads C2Cl6. That’s two carbons bound up with six chlorines, nothing fancy at first glance. I remember jotting it down in high school, feeling the clean symmetry of those repeating Cls. But the formula only scratches the surface. Chemical structures like this often turn up in places we don’t expect—military smoke grenades, old-time fire extinguishers, even in some metal casting processes. Hexachloroethane’s journey from raw material to finished product deserves a closer look than a desk copy of a chemistry book can offer.
Where the Formula Shows Up in Real Life
C2Cl6 isn’t something you stumble across in the kitchen or garage. It mostly turns up in industrial settings. Foundries use it to help remove oxygen from molten metals. Pyrotechnic mixes use it to make dense white smoke. In my time around foundry workers, I’ve listened as they shared concerns about fumes from compounds like this, especially indoors. Hexachloroethane creates thick clouds for concealment or as a signal, so safety matters.
Safety Talks: Health and Environmental Impact
Plenty of studies outline problems with exposure to chlorinated solvents like hexachloroethane. Short bursts of inhalation can cause headaches and nausea. Longer exposures—or being in the wrong place at the wrong time—lead to liver and kidney trouble, irritation in the lungs, and sometimes something worse. Chronic exposure in poorly ventilated industrial environments has left its mark on workers over the decades.
Runoff containing C2Cl6 doesn’t just stop at the property line; rivers and soil can carry traces for years. Wildlife studies regularly flag chlorinated chemicals in fish and birds living near larger facilities. Some government agencies restrict its use, laying out how much can be present in factory air or local water supplies. Recent research shows a clear push toward finding alternatives wherever possible, and plenty of safety protocols demand close monitoring.
Fine-Tuning Practices: Rethinking How We Use C2Cl6
In my working life, I’ve seen old products phased out with the help of better valves, tighter controls, better waste handling, and—sometimes—better substitutes. For hexachloroethane, using less, using safer alternatives, or applying closed systems helps protect workers. Facilities that upgrade their ventilation systems and give regular safety talks see fewer incidents. Routine monitoring and medical checks for workers help catch health problems early.
Regulators and scientists both push for more transparency. Strong data collection and whistleblower protections encourage workers to speak up if they notice problems. Third-party audits catch corners cut for speed or cost. This kind of accountability, where facts guide decisions, pushes the industry forward. I’ve watched organizations bring down emissions and protect both workers and neighbors with the right mix of oversight and honesty.
Moving Forward with Awareness
Chemical formulas—like C2Cl6—fit on a single page, but the details matter out there where people work. Getting experience with these materials firsthand, seeing how practices on paper play out in real workplaces, adds a layer of understanding that no quick lookup can match. The push to keep workers safe and the environment a little cleaner only gets stronger as the facts come out and the stories get told.
Understanding the Risks of Hexachloroethane
Most folks outside of chemical plants or the military might not bump into hexachloroethane at the hardware store, but all kinds of industries use it—for smoke bombs, metallurgy, and some lab processes. Hexachloroethane doesn't belong in household trash. It’s heavy on chlorine, known for toxic fumes and environmental hazard. This stuff hangs around in soil, leaches into groundwater, and doesn’t play nice with wildlife or human lungs. I remember seeing workers at a foundry taking extra care with even sealed containers—they all knew a single spill could mean trouble for the water table.
Government agencies like the EPA and OSHA flag hexachloroethane as a hazardous waste—so tossing it in a dumpster just spreads trouble. Its disposal brings up the kind of lessons folks in small-town America learn after seeing a local creek poisoned by illegal dumping. Once it’s out in the wild, cleaning it up costs a bundle and leaves scars for decades.
Proper Disposal: No Shortcuts
For safe disposal, communities rely on hazardous waste programs, not landfills. Licensed hazardous waste handlers know the ropes. They have sealed drums, ventilated trucks, and hazmat-trained teams. At the disposal facility, high-temperature incinerators handle hexachloroethane—burning it away at temperatures above 1,200 degrees Celsius. Most backyard setups can’t reach those numbers and, honestly, nobody wants that smoke drifting into the next county.
Some companies contract with professional chemical recyclers to handle leftovers. Chemical recycling cuts the danger of leaks and leaching. Facilities filter emissions, scrub out the worst pollutants, and track every barrel to its final fate. Anyone working in labs or foundries learns early: you call the professionals, fill out the chain-of-custody paperwork, and don’t cut corners.
Regulations and Community Responsibility
EPA guidelines paint a clear line: improper disposal can carry stiff fines. I’ve seen local governments invest in annual hazardous waste collection events to help regular folks clear out cabinets safely. It also helps educate—most people have no idea what's lurking behind paint cans in the garage. Industry insiders and municipal governments need to up the outreach and make these services easy to use.
No one wins when shortcuts rule. Some older facilities used to send waste to landfills, and the result was contaminated groundwater that still shapes health decades later. Strict records, transparent reporting, and traceable disposal all add up to fewer mistakes. Personal responsibility shows up, too—I used to join local environmental groups that pushed for bigger budgets and more oversight, because one mistake quickly becomes everyone’s problem.
Finding Real Solutions
Safe disposal depends on strong laws, honest companies, and community pressure. Facilities should adopt better tracking systems so every ounce of hexachloroethane gets accounted for from doorstep to furnace. Cities can double down on public drop-off days and run outreach campaigns for schools and businesses. At a personal level, reading the label and asking professionals before handling any unfamiliar chemical beats trying to Google homebrew fixes. Less hexachloroethane in the wrong place means cleaner air, safer water, and fewer health headaches for everyone down the line.