Methyl Dichloroacetate: Unpacking a Chemical That Shapes Modern Science
Historical Development
Methyl dichloroacetate didn’t just show up in labs overnight. Chemists first explored the broader class of dichloroacetates in the late nineteenth century when chlorine chemistry started booming. Early work on halogenated carboxylic acids got people curious about their reactivity and practical use, and over time, as organic synthesis advanced, researchers nailed down methods to prepare pure methyl dichloroacetate. Interest picked up in the twentieth century because this molecule showed promise in both industrial chemistry and biomedical fields. Its development evolved alongside improvements in separation, characterization, and environmental awareness; old synthesis techniques produced byproducts that today we’d struggle to justify on safety grounds, but lab safety back then rarely got top billing. Now, scientists draw on decades of accumulated know-how to shape how methyl dichloroacetate is produced, used, and regulated.
Product Overview
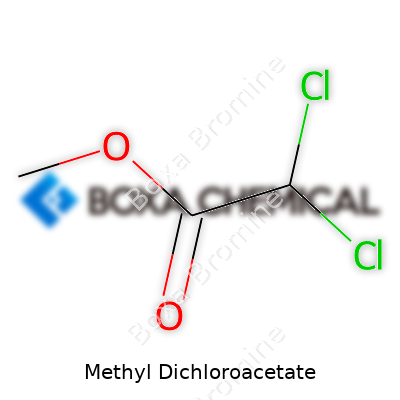
Methyl dichloroacetate stands out as an ester derived from dichloroacetic acid, with a sharp, sweet odor reminiscent of many chlorinated organics. You usually spot it as a clear, sometimes barely yellowish liquid, packaged in tightly sealed bottles. It finds its place in analytical chemistry, synthesis of specialty chemicals, and occasionally crops up in pharmaceutical research labs seeking out unique intermediates. I’ve watched how chemists reach for this molecule when they need reliable reactivity—chlorinated esters don’t just sit around; they engage with plenty of compounds, which makes methyl dichloroacetate a staple for experimental design.
Physical & Chemical Properties
You won’t forget the smell if you’ve worked with methyl dichloroacetate—acrid and chemical, proof of the two chlorine atoms lurking in each molecule. It boils at roughly 129°C, well below water’s mark, and mixes decently with organic solvents such as ether, chloroform, and benzene, but keeps its distance from water. Its molecular formula reads C3H4Cl2O2, clocking in with a molecular weight near 143.97 g/mol. Like many chlorinated compounds, it throws around enough volatility to demand careful handling. Chemists value the ester’s resilience in many processes—this molecule stays put across a range of pH environments, and its chemical bonds deliver the stability needed in tough reaction conditions.
Technical Specifications & Labeling
On the shelf, amber glass bottles keep methyl dichloroacetate safe from light. Product labels give concentration, purity (usually 98% or greater for research applications), and hazard warnings like flammability and toxicity. Some bottles carry QR codes now, linking to batch certificates and safety sheets—this lets researchers double-check spectra and impurity profiles. That kind of transparency matters: in my experience, a few percentage points of unknown material can throw whole experiments off kilter. Packaging often meets international shipping standards; no reputable supplier cuts corners with a material this reactive.
Preparation Method
Making methyl dichloroacetate takes controlled steps. Most routes start with dichloroacetic acid and methanol using acid catalysis—the Fischer esterification process at its core. Sulfuric acid usually pushes this reaction forward, pulling water out and letting the methyl ester form. The reaction runs at moderate temperatures under reflux, the product distills out, and then purification steps drive off leftover methanol and acid. Industrial processes may vary, but careful control at each stage guarantees the trade-off between yield and safety. At bigger scale, chemists switch to continuous distillation setups to dodge buildup of flammable or corrosive gases. I’ve seen reactions scaled up safely by engineers who never lose sight of venting and material compatibility.
Chemical Reactions & Modifications
Methyl dichloroacetate’s value lies in its unique reactivity. Those two chlorine atoms don’t stay untouched for long; nucleophilic substitution reactions allow crafty chemists to swap them for more interesting groups. The ester linkage stands up to a surprising number of conditions but can be hydrolyzed with strong acids or bases to yield dichloroacetic acid. In the right hands, it forms new amides, ethers, or other esters. Medicinal chemists find it handy to tweak molecular scaffolds during drug development. Organic labs regularly divert this compound toward new polymers or as a component in agrochemical syntheses. What really grabs attention for researchers is its ability to undergo select cross-coupling reactions for more targeted modifications.
Synonyms & Product Names
Methyl dichloroacetate appears in lab catalogs under a handful of trade names and synonyms. Some folks call it dichloroacetic acid methyl ester, others opt for simple abbreviations like MDCA. In commercial settings, brand names rarely matter as much as purity and source; researchers want lot data and chemical certifications, not catchy names. Still, global suppliers list "methyl dichloroacetate" as the universal tag, smoothing cross-border procurement. In technical documents and publications, the IUPAC name, methyl 2,2-dichloroacetate, leaves no room for confusion, no matter your country or language.
Safety & Operational Standards
Using methyl dichloroacetate never feels routine, because strict safety standards frame every step. The product belongs to both flammable and toxic classes; it’s handled only in ventilated hoods, with gloves and eye protection as the norm. In the lab where I trained, we locked the bottle in dedicated cabinets with secondary containment, and every transfer required clear documentation. Technicians know spills become emergencies because even minor contact can irritate skin or eyes, and inhalation risks demand extra caution. Disposal runs through licensed waste streams—pouring down a drain sits off the table. On the industrial side, chemical-resistant piping, overflow alarms, and remote monitoring protect workers and facilities. Safety data sheets get reviewed not just on first use, but with every new task. It takes that level of care to prevent accidents, especially with smaller teams or new hires.
Application Areas
I’ve seen methyl dichloroacetate prove its worth in arenas from pharmaceutical chemistry to specialty solvent production. Its main claims come from work as both a building block and a reagent—researchers synthesize new drug candidates, biochemists use it to probe metabolism, and industrial chemists find value crafting advanced plastics or pesticides. There’s even ongoing exploration of using it in environmental analysis for monitoring chlorinated compound residues. Product engineers hunting for high-performance intermediates land on this molecule for its unique blend of volatility and functional group placement. Every field that leans on synthetic chemistry can name at least a few projects that lean on methyl dichloroacetate.
Research & Development
Labs across the world have pushed methyl dichloroacetate into new frontiers. Some focus on tuning its reactivity; others study environmental fate and breakdown pathways. Academic groups search for greener synthesis using biocatalysts or alternative solvents, trading harsh acids for milder, more sustainable conditions. Biomedical researchers eye this compound for its metabolic effects, and more than a few papers have probed potential cancer therapies leveraging its ability to modulate certain enzyme systems. In my experience, regulatory pressure keeps research honest—any promising pathway must contend with thorough scrutiny from both safety and efficacy points of view. That means grants fund not just “can we make this,” but “can we do it at scale without unacceptable risk.”
Toxicity Research
Toxicologists don’t take methyl dichloroacetate lightly. Animal studies show it affects the liver and kidneys when exposure gets too high, and chronic studies keep an eye out for carcinogenicity. Researchers map out safe exposure ranges for both acute and chronic contact, and most protocols treat this molecule as a chemical hazard needing proper engineering controls. The EPA and European agencies set clear workplace standards and environmental discharge limits. Researchers keep pushing to know what breakdown products form in soil, water, and living tissue—without real data, regulators won’t budge. I’ve seen teams test new cleanup agents so accidental releases never threaten drinking water or wildlife, and from where I stand, this vigilance protects more than the people in the lab.
Future Prospects
Looking ahead, methyl dichloroacetate still attracts both excitement and scrutiny. Some experts see a future in cancer therapy, if researchers can separate toxic side effects from genuine clinical benefits. Industrial users want to push yields higher while keeping emissions and waste in check, and ongoing studies try to swap out petroleum-based starting materials for more sustainable feedstocks. Green chemistry takes center stage—biocatalytic and photochemical approaches line up as promising alternatives. The field faces plenty of regulatory hurdles, especially for consumer or pharmaceutical products, but the chance to find less hazardous derivatives motivates continuous improvement. I think the next decade will bring both safer processes and new uses we haven’t yet imagined. That’s not hope—it’s a lesson from history: each wave of research builds on what came before, and good stewardship keeps dangerous mistakes from repeating.
Tracing Its Presence Across Industries
Methyl dichloroacetate shows up in places most folks never think to look. With its chemical structure closely related to other dichloroacetates, industry has found several uses for it, especially thanks to its role as a reagent. Chemical manufacturers tap into its abilities when they need a selective chlorinating agent. In the world of specialty synthesis, nothing replaces a tool that introduces two chlorine atoms so precisely.
Roots in Research and Manufacturing
Researchers depend on methyl dichloroacetate for making other compounds. Its unique mix of reactivity and stability means labs reach for it when seeking to build more complex molecules, or adjust existing structures for tailored performance. Although pure research may feel worlds away from everyday life, the breakthroughs depend on starting materials like this one.
Factories making flavors, fragrances, and some pharmaceuticals sometimes draw from methyl dichloroacetate’s toolkit. For example, custom intermediates may require putting the dichloroacetate group into place. It’s not an ingredient found in consumer goods directly—more like a stepping stone that vanishes once the path is built.
Why Handling Demands Respect
Anyone who’s spent time around chemical processes knows that compounds with multiple chlorines usually bring risks. Methyl dichloroacetate cannot be handled carelessly; inhaling or spilling it could cause real harm. Occupational safety data warns about its toxicity, linking exposures to organ damage in the worst cases.
Proper handling gear makes all the difference. Good ventilation, gloves, and tightly controlled procedures stand between safe operations and emergency room visits. Regulatory bodies in Europe, North America, and Asia write strict workplace rules for it. The sheer number of protocols illustrates how important it is to keep risk in check.
Turning to Solutions for Safer Work
The challenges with methyl dichloroacetate call for more than just compliance—they call for real commitment to best practice. In my years consulting for chemical plants, I’ve seen the stark difference safety culture makes. Trained workers, up-to-date information, and clear spill responses prevent disasters before they begin.
Switching to less hazardous substitutes deserves attention too. Pharma and agrichemical sectors have begun rethinking foundational reagents in their pipelines. It takes investment, but finding alternatives where possible gives long-term payback in both health and environmental terms. Not every process can abandon methyl dichloroacetate overnight, but pressure from regulators and conscious companies nudges things in that direction.
Looking Ahead
As the world pushes for greener chemistry, every step away from high-toxicity intermediates matters. Methyl dichloroacetate’s story serves as another example of how technical solutions intersect with real-world safety and ethics. Industries willing to question old recipes—backed by fresh research and rigorous safety—will find themselves ahead as regulatory and social expectations climb higher.
The Substance in Focus
Methyl dichloroacetate doesn’t get much mention outside chemical circles, but it grabs attention for a reason. With the rise of niche applications, people ask if it poses a risk in the workplace or lab. Truth is, every solvent or reagent needs a healthy amount of respect, and this one deserves no less. I’ve spent years in industrial labs, where familiarity with substances like methyl dichloroacetate never breeds complacency.
The Hazards Up Close
To start, you won’t need to dig deep to uncover health warnings about methyl dichloroacetate. Straight from safety data sheets, direct contact irritates skin and eyes. Breathing the vapor can bring headaches or dizziness. Chronic exposures in lab settings sometimes lead to more serious symptoms. The substance can enter through skin or inhalation, ending up in the body—which the human system won’t appreciate.
Researchers have tested its effects in animals. Some studies point to liver toxicity after repeated exposure. Even if those studies use high doses, nobody in a responsible facility shrugs off such findings. The National Institutes of Health lists methyl dichloroacetate as a hazardous material. A peer-reviewed study in the journal “Toxicology” highlights possible nerve toxicity in lab animals. That’s enough incentive to minimize all direct and indirect contact—no one wants to test the limits on themselves.
Everyday Handling Scenarios
People encounter chemicals all the time, sometimes under the wrong assumption that lab-scale equals low risk. In a manufacturing plant, even an ounce spilled without proper gear quickly becomes a problem. I’ve seen colleagues underestimate the volatility of similar compounds. Ventilation evaporates risk much slower than most would expect. One slip, one poorly sealed bottle—suddenly someone feels woozy or winds up with a chemical burn.
Personal protective equipment matters here. Not just gloves—nitrile or better—but also full splash goggles and a face shield for transfers. Labs with decades of spotless records rarely make exceptions on these points. Splash-proof lab coats and closed shoes outperform streetwear every single time. Nobody regrets wearing too much, only too little.
Safe Practice in Storage and Disposal
Storing methyl dichloroacetate alongside common flammables sets the stage for trouble. The compound reacts with strong bases or oxidizers. Any spill near incompatible chemicals ramps up the probability of an emergency. I’ve seen colleagues lock down chemicals in separate ventilated cabinets, far from heat or sunlight. Every company I worked with logged inventory closely and kept containers labeled. That cut down on accidents caused by mistaken identity or improper mixing every year.
Disposing methyl dichloroacetate can’t go down the drain with ordinary lab liquids. It asks for designated waste streams routed to hazardous waste contractors. Attempts to shortcut this process create environmental headaches. Some local governments issue heavy fines for improper handling. Responsible labs cooperate with professional services—protecting waterways is about community safety, not paperwork.
Learning and Training—The Real Barrier
Trust grows from repeated training and a culture that expects caution. The new technician gets the same lecture as the old pro; no one skips the safety walkthrough. Posters listing emergency showers and spill kits act as reminders, not decorations. I’ve worked on teams where peer checks were standard—correcting a glove error or a face shield slip before the first bottle even opens. In those environments, incidents stick out as rare exceptions. That keeps everyone healthier and sleeping better at night.
The Formula and What It Reveals
Methyl Dichloroacetate comes with a straightforward chemical formula: C3H4Cl2O2. This formula isn’t just a string of letters and numbers tossed together. Each symbol stands for something real—carbon, hydrogen, chlorine, and oxygen, all arranged to build a compound that has roused curiosity in labs and industries. There’s more to this than just assembling pieces. The arrangement of these atoms makes this molecule unique, giving rise to its specific properties and real-world uses.
Why Learning Chemical Formulas Matters
Ask anyone who has spent time mixing solutions in a classroom, or someone burning the midnight oil in pharmaceutical research, and you’ll hear this answer: understanding a chemical formula paves the way to predict everything from boiling point to biological behavior. I've seen firsthand how underestimating a chemical’s potency or reactivity can backfire. In an industrial setting, treating C3H4Cl2O2 like just another solvent would be a mistake, because those chlorines can make the compound more reactive and even hazardous in certain cases.
Impact on Industry and Medicine
This compound has drawn attention in several fields. Researchers have looked at Methyl Dichloroacetate for its role in metabolic therapy and some experimental cancer treatments. It’s not a household name yet, but curiosity around this molecule is growing. People in pharmaceuticals and chemical manufacturing can’t afford a surface-level understanding. Mislabeling a drum, misunderstanding a formula, or skipping a safety step because of rushed research can cause harm, both to employees and the surrounding environment. Chlorine atoms in a molecule signal potential toxicity and demand respect.
Safety is Always Part of the Equation
Having spent time in both the lab and on the factory floor, I can say that the conversation always comes back to safety. C3H4Cl2O2 is a volatile molecule. It doesn’t just sit there like a napping cat. Spill some, mix it accidentally with the wrong reactive agent, or bypass a fume hood, and risks escalate fast. Many industrial accidents can be traced to moments where people lost sight of chemical specifics and basic protocols. Every digit and atom in this formula carries weight. Training staff, clear labeling, and robust emergency plans stop dangerous mistakes long before they happen.
Transparency and Ethical Handling
Moving forward, everyone involved with chemicals—manufacturers, researchers, teachers—benefits from speaking openly about risks and complexities. Public trust depends on it. I remember times when confusion around chemical names caused delays or even led people to buy the wrong material. Educational campaigns that demystify formulas, regular safety refreshers, and honest documentation serve both the expert and the newcomer. Companies living up to these standards demonstrate commitment to both safety and integrity, two things that matter in every chemical conversation.
Navigating Future Challenges
Progress in chemical sciences leans on two pillars: deep knowledge and solid safety culture. The formula C3H4Cl2O2 might look dry on paper, but it represents an entire chain of responsibility. As the demand for specialty chemicals grows, a culture of respect and rigor becomes critical. Companies could adopt digital tools to track chemicals, hold regular in-house workshops, and support open lines of communication between management and workers. Risks drop, confidence goes up, and the world becomes a bit safer with every correctly read formula.
Looking Past Labels and Into Real Storage Challenges
People who work with Methyl Dichloroacetate, whether in a small lab or an industrial setting, know the hazards are real. One careless mistake can bring serious consequences. This chemical, with its pungent smell and strong reactivity, brings safety risks if handled without thought. I’ve seen plenty of accidents in research environments that could have been avoided by simple storage steps. Sometimes, it’s not the handling, but what happens when the bottle sits for months on a shelf. Vapors leak, labels fade, shelves crowd—these add up to hidden dangers that sneak up on anyone who skips the basics.
Temperature and Container Matter Far More Than Most Guess
Methyl Dichloroacetate isn’t friendly with moisture. Storing it anywhere near water opens doors to slow degradation, or worse, surprising reactions. Shelves in humid basements, old wooden cabinets that soak up ambient air, and even a busy chemistry prep room all invite trouble. The right storage calls for a cool, dry spot—think sealed flammables cabinet or a climate-controlled storage room. Stainless steel or high-grade glass containers keep things stable. Low-density plastics never last long here. I’ve learned this firsthand while auditing chemical storerooms: poorly matched containers warp or leach, risking leaks.
Ventilated Spaces Fight Fume Buildup
Sealed tight, this stuff still gives off fumes over time, especially if the seal isn’t perfect. I never trust a shelf tucked away in an unused corner or in a locked closet without airflow. Proper chemical storage needs ventilation. Fume hoods or dedicated storage cabinets, pulled into a negative pressure system, help keep breathing zones safe. Colleagues who’ve attempted shortcuts—sticking bottles behind other supplies—often regret it when they discover odors creeping past sealed doors. The right practice scents out small leaks before they pose a bigger threat.
Clear Labeling Keeps Big Problems at Bay
Even when a chemical sits idle, constant shifts in staff and seasons invite confusion. Years back, I helped clean an old university storeroom. We found a dusty bottle with a label rubbed almost blank, with only a faded streak of “dichloro…” left. Keeping everything clearly labeled with the full name, date of purchase, and concentration saves headaches. Labeling systems using color codes or barcodes make inventories quicker and mistakes far less likely. A rushed moment never justifies grabbing an unlabeled or mystery container. The disaster stories all started this way.
Fire Risks Demand Respect and Smart Placement
Methyl Dichloroacetate brings a flammable risk. Storing it away from open flames, heat sources, and oxidizers is non-negotiable. I recall seeing an overloaded shelf above a radiator, packed with chemical bottles—an invitation for disaster. Good storage means sturdy shelving, enough spacing to avoid bottle collisions, and no heat sources nearby. Fire-resistant cabinets rated for flammables give the best insurance against both spills and bigger emergencies. Quick access to chemical spill kits and fire extinguishers rounds out a safe setup.
Improving Safety: Small Steps With Big Impact
Some think strict rules only slow down work. My own experience points the other way: strong storage practices actually save time and trouble. Regular audits, honest risk assessments, and ongoing training bring calm to busy labs. Posting safety sheets near storage, using spill trays, and rotating stock so nothing goes forgotten all strengthen a smarter culture. As work in science and industry keeps moving faster, these basics give peace of mind.
Understanding What Methyl Dichloroacetate Does in the Body
Methyl Dichloroacetate has been around lab benches and industrial sites for years, but most people have never heard of it. Once it’s in the air, on your skin, or swallowed by mistake, this chemical gets inside your body and heads straight for your liver. Based on research from the National Institutes of Health, one thing stands out: the liver sees the worst of it. This organ works hard to process almost everything that ends up in your bloodstream. Instead of breaking this compound down easily, the liver struggles, sometimes leading to inflammation or cellular damage.
A few bad headaches or some nausea after exposure sounds manageable, but studies highlight more than that. Animal tests point toward nerve problems and even some heart muscle effects. Workers who have spent enough time around chemical plants talk about odd tingling, fatigue, or losing their sense of balance. Research suggests those symptoms likely didn’t just come out of nowhere. Bodies under chemical stress from substances like Methyl Dichloroacetate can show these warning signs well before blood tests look unusual.
Looking At Cancer and DNA Risks
Scientists always ask if a man-made compound could start cancer, and for good reason. Chronic exposure sometimes brings up the “C” word. Lab studies have pushed rats and mice to the limits, and some link this chemical with increased tumor rates, especially in the liver. Researchers haven’t found direct, conclusive evidence in people, yet regulatory agencies take the animal data seriously. They flag this substance as having possible cancer risks.
DNA doesn’t deal well with chemical attackers. In some studies, cells exposed to Methyl Dichloroacetate showed DNA changes. Consistent DNA hits from toxins over time can set up a person for more problems down the road. No one wants to roll the dice on mutations, especially near places where families live, work, and play.
What Workers and Communities Can Do
Cities sprouted next to industrial plants before anyone talked about “environmental justice.” Some neighborhoods still face heavy chemical traffic because of old zoning maps. For workers, gloves, face shields, and good ventilation go a long way. I’ve worked warehouse jobs surrounded by drum-lined chemicals, and it’s clear that quick showers, double washing work clothes, and smart storage cut risk. Managers should make air monitoring a priority, since invisible vapors are sometimes the biggest threat.
Public health nurses in chemical-heavy towns sometimes organize yearly health checks. They track symptoms before anyone even has a diagnosis. That’s prevention in action. I’ve seen local councils push industry for fenceline air sensors and emergency shelters. When residents know what’s in their air or water, they push for change faster. Opening up data to the public doesn’t just build trust — it saves lives.
Boosting Oversight and Accountability
Real change comes with stricter regulations. Agencies like OSHA and the EPA can require companies to post their emissions and keep exposure below clear safety lines. If companies dodge those responsibilities, hefty fines move the needle. Medical surveillance among exposed workers — regular checkups, blood testing, early nerve screening — should never be optional.
Many chemicals, including Methyl Dichloroacetate, bring benefits in research and manufacturing, but blind spots and shortcuts set up communities and workers to pay a steep price. Balancing industrial progress with human health takes more than paperwork — it’s hands-on work, transparent data, and constant vigilance.