Chlorotrifluoroethylene: A Deeper Look
Historical Development
Chlorotrifluoroethylene’s story started in the early twentieth century with the rise of organofluorine chemistry, a field that transformed manufacturing, aerospace, and medicine. During the late 1930s and the Second World War years, researchers scouted for new refrigerants and solvent bases, driving attention to halogenated olefins like chlorotrifluoroethylene. By the 1940s, the chemical had moved from bench experiments into industrial reactors. Its unique properties—mostly its high chemical resilience and low flammability—stirred up excitement among firms looking for durable coatings and specialty polymers. Chemists like those at DuPont kept refining the production process, paving the way for related polymers such as polychlorotrifluoroethylene (PCTFE), which soon found an audience in demanding sectors. This innovation did not arrive without growing pains; manufacturers scrambled to refine handling protocols and assure health and environmental safety before regulators imposed sweeping rules.
Product Overview
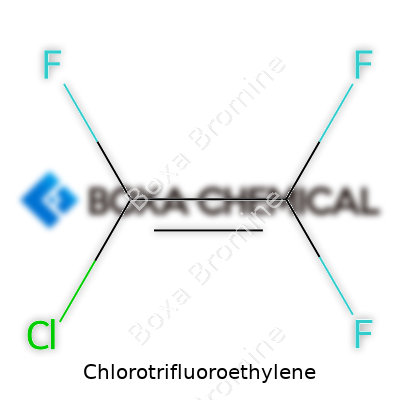
Chlorotrifluoroethylene appears as a colorless gas with a faintly sweet odor, far from the average person’s daily experience but critical in high-tech industries. Its chemical formula—C2ClF3—captures a combination of chlorine and fluorine around an ethylene core, making it an unusual candidate for processes that need inert or durable materials. In production plants, it ships pressurized in sturdy steel cylinders, often marked with the chemical’s trade names—sometimes Fluorothene or Halocarbon 1113. The price and demand keep rising as the market expects high-performance coatings, wire insulations, and barrier films, all depending on the exceptional qualities this compound delivers.
Physical and Chemical Properties
The gas can withstand wide swings in temperature—boiling at about -32 °C and remaining stable under heavy pressure. It carries a density just above air, which shapes the choice of storage and transport. Chemically, the molecule’s strong carbon-fluorine bonds resist attack from acids, bases, or most solvents. Industry people know its weakness: exposure to sparks, flames, or ultraviolet light can trigger violent reactions, producing toxic gases. Its chemical inertia serves as both an advantage and a curse; while decomposition is rare, the products are notorious for safety risks. This stubborn molecule resists corrosion, dissolves in organic solvents, but stays largely unmoved by water, which is precisely why firms pouring millions into high-spec barrier films often call for it.
Technical Specifications and Labeling
The gas usually ships in steel tanks under pressures up to 40 bar. Cylinder labels flag its UN identification: UN 1081, marked for “Compressed Gas, Flammable, N.O.S.” Manufacturers print hazard diamonds and detailed safety handling directions straight onto the labels, highlighting the risk of inhalation and the need for leak-proof handling. It earns high rating for its “zero” ozone depletion potential, but gets marked “danger” because of its fire-promoting behavior under heat or electrical discharge. Today’s regulations—enforced by OSHA, the EPA, and their foreign equivalents—require transparent documentation covering content, batch information, and explicit guidance for emergency response should leaks or ruptures occur at the warehouse, rail yard, or laboratory.
Preparation Method
Large-scale plants use the dechlorination of chlorotrifluoromethane or the pyrolysis of trichlorotrifluoroethane over catalysts. Commercial routes tend to favor passing chlorotrifluoromethane over a heated metal catalyst, squeezing out bountiful yields under controlled pressures and temperatures. In practice, technicians calibrate reactor temperature and residence time to stamp out impurities—especially highly reactive byproducts like hexafluoropropene—that clog downstream systems. Each step draws on decades of fine-tuning, a legacy of laboratory and plant mishaps that trained practitioners to respect this gas and master its safe collection and storage.
Chemical Reactions and Modifications
Alone, the gas stands inert to many reagents, but heat or free radicals crack open the double bond, allowing addition or polymerization. Producers harness this feature by triggering radical polymerization, chaining the monomers into PCTFE, an engineering plastic with unmatched resistance to weathering, chemicals, and ultraviolet light. Chemists sometimes copolymerize it with other halogenated olefins for specific strengths or flexibility, customizing the end product for aerospace or electronics. The reactivity goes beyond polymers: researchers coax the molecule to yield specialty intermediates by controlled reactions with nucleophiles or bases, birthing entirely new classes of performance materials from the same sturdy building block.
Synonyms and Product Names
Depending on the market or the application, chemical suppliers list chlorotrifluoroethylene by names like CTFE, 1-chloro-1,2,2-trifluoroethylene, or sometimes, simply “trifluorochloroethylene.” Trade catalogs note Halocarbon 1113 or Fluorotene, each denoting tailored blends or degrees of purity. Buyers scrutinize these listings, pressing vendors to clarify grade and intended use since electrical, food, and medical applications demand tighter control of impurities compared to general-purpose polymer feedstocks.
Safety and Operational Standards
Anyone who has stepped into a chemical plant remembers the drill: strict ventilation, spark-free tools, and multi-level pressure monitoring wrap every encounter with chlorotrifluoroethylene. Workers suit up in flame-retardant gear, with escape hoods close by. Regulations like the U.S. Occupational Safety and Health Administration’s (OSHA) PEL, Europe’s REACH, or Japan’s ISHL govern every shipment, mandating air monitoring and reporting. Facilities must maintain leak detectors, emergency shutoffs, and fire suppression—nothing gets left to chance. Training isn’t a box-checking exercise; it’s a lived reality, reinforced with drills and incident debriefs. Only people with hands-on competency get clearance to manage this compound.
Application Area
Polychlorotrifluoroethylene, born from this monomer, turns up in high-performance seals, gaskets, wire coatings, and medical blister packaging. Its chemical backbone shrugs off acids and bases, keeping corrosive leaks at bay in chemical plants. The film’s ability to form vapor barriers gives pharmaceuticals a longer shelf life and clean rooms more reliable protection against moisture. Electronics makers lean on CTFE polymers for insulation that doesn’t degrade under harsh loads. Aerospace designers count on it to guard delicate gears and circuits from the brutal assaults of high-altitude cold and radiation. Its value stretches into novel uses too, like micro-pore membranes and oxygen sensors, where conventional plastics buckle under stress.
Research and Development
Labs keep probing for ways to tame and shape CTFE’s potential. Better copolymers, new catalysts, and enhanced safety in processing all stand out in the latest research. Papers detail efforts to improve melt processability and to cut environmental footprints with efficient recycling or safer alternatives. Researchers at top universities dive into the physicochemical behaviors under extreme conditions to decode long-term durability. Improving scalability while reducing hazardous emissions forms the cutting edge, and grant money increasingly flows into green chemistry routes and lifecycle analysis, reflecting society’s push towards sustainable industry. Direct experience shows how collaboration between industry chemists and academic researchers keeps the innovation pipeline open—and keeps mistakes from repeating themselves.
Toxicity Research
Toxicologists take chlorotrifluoroethylene’s risks seriously. Inhalation triggers dizzy spells, headache, and, at higher exposures, unconsciousness or worse. Chronic exposure links to kidney and liver issues in animal models, prompting close study of excretion pathways and metabolic byproducts. The fluorinated structure means bioaccumulation rates are generally lower than older organochlorines, but uncertainty remains about low-level, long-term public risks. Regulatory studies drive stricter workplace exposure limits. Medical surveillance, atmospheric monitoring near plants, and animal studies anchor safety protocols, with each incident or new finding steering policy. Younger researchers see firsthand how a single mishap or misreading can ripple through a workforce or a neighborhood, keeping safety front and center.
Future Prospects
The push for greener, safer materials may challenge CTFE chemistry in the coming decades, but its core strengths—endurance, resistance, and versatility—mean demand will not disappear. Polymer scientists eye new blends that reduce fluorine content, easing environmental handling but holding on to performance. The circular economy concept influences R&D, nudging industry to close the materials loop through recycling or reuse, not just one-way consumption. Start-ups and global chemical players alike pursue catalysts that slice energy use in prep stages, guided by climate pledges and stricter compliance standards. Safe operation and rigorous environmental stewardship may not grab headlines, but those priorities hold the key to sustained industrial trust in this remarkable compound.
What Really Makes Chlorotrifluoroethylene So Useful?
Chlorotrifluoroethylene, often shortened to CTFE, shows up in more places than a lot of people realize. I got curious about it while trying to fix a leaky valve in my neighbor’s garden hose. The plumber who came by mentioned some industrial plastics made from CTFE, and that set me down a rabbit hole. It’s used mainly as a building block for polymers like polychlorotrifluoroethylene (PCTFE), which tackles tasks that go beyond the reach of everyday materials like nylon or polyethylene.
Why Do So Many Industries Turn to CTFE?
Companies need materials that can handle tough environments. Pipes, cables, coatings, and seals made with PCTFE from CTFE resist some of the harshest chemicals and extremes in temperature. The Arctic gets pipes lined with this polymer. Labs handling dangerous acids or strong solvents pick PCTFE parts since they don’t fall apart or corrode over time. Valve seats, gaskets, and o-rings are often made from this material, especially in aerospace. You don’t want a risk of failure at 35,000 feet, and that’s where CTFE-derived plastics give confidence.
Packing and Food Storage
One of the less obvious things I found is that this compound guards freshness in food packaging. PCTFE films keep out moisture better than almost anything else. Pharmaceutical companies use it to keep pills dry and stable on long journeys. Food manufacturers want their snacks crispy and medication folks want reliable shelf life—PCTFE films deliver both. I once stored crackers in a regular bag and they went stale in days. A CTFE-based film keeps that from happening, even in humid climates.
Protecting Sensitive Electronics
PCTFE stands out in electronics where parts can’t rust or break down from exposure to water vapor. I remember building a weather sensor project for a science fair, and moisture nearly ruined one of the components. Insulating cases made with CTFE polymers shield delicate circuit boards, helping them run longer and safer. High-reliability cables—especially under the sea or out in space—often use this insulation, blocking both water and aggressive chemicals.
Safety and Environmental Facts
Plastics from CTFE don’t ignite easily. In aircraft or nuclear power plants, fire can mean disaster, so flame resistance ranks high on the priority list. The material also produces little smoke, which helps in emergency situations. Longevity matters, too. Reducing the need for frequent replacements cuts down on plastic waste over time. It's worth thinking about the recycling challenges, though. PCTFE is tough to break down and reuse, which circles back to the need for industry to develop better disposal or recycling strategies.
How Could Things Improve?
Efforts should focus on safer production, handling, and ultimately, waste management of CTFE-based plastics. Factories need to step up on leak prevention and worker safety, since the raw chemical has health risks if inhaled. Directing research at creating bio-based or more readily recyclable alternatives would help, but real-world performance still leans on CTFE for some irreplaceable tasks. As the call for sustainability grows louder, teams in both industry and academia have to look at extending product lifespans, improving manufacturing processes, and finding post-use pathways so CTFE’s benefits don’t overshadow its environmental footprint.
Why Precautions Matter
Chlorotrifluoroethylene doesn’t get much press, but it’s common in factories that make plastics and specialty coatings. Some people treat chemicals like this as just another unpronounceable name on a label, but the truth runs deeper. Exposure to chlorotrifluoroethylene (CTFE) gas can cause breathing problems, skin burns, and eye irritation. In all my years working in and visiting chemical plants, the folks who treated safety measures as suggestions, instead of rules, faced more than their share of accidents. CTFE isn’t one of those chemicals you can shrug off, mainly because once it vaporizes, it spreads quietly through air and lingers almost undetected.
Essential Gear and Air Monitoring
The right protective gear makes a world of difference. Gloves, goggles, and chemical-resistant suits act as your first line of defense. Forgetting any of those can quickly turn a routine task into a trip to the emergency room. Since CTFE is heavier than air, it tends to collect in low spots—trench drains, pits, and sumps. Air monitoring matters here. Using real-time gas detectors near ground level means you know if the stuff is beginning to pool, before anyone gets exposed. Relying on just nose or instinct almost always leads people astray.
Handling Leaks and Spills
Leaks may sneak up on you. From what I’ve seen, nobody ever expects to discover a dripping valve right before quitting time, but it happens. Having a clear plan gives workers confidence to react, instead of freezing on the spot. Evacuate the area, ventilate if possible, and alert the emergency response team. Most facilities with frequent CTFE use keep spill kits stocked with absorbent pads, neutralizers, and a map showing the quickest route out. Clear signage and unblocked exits save lives. It doesn’t matter whether the leak is a slow drip or a sudden gush — treating every spill seriously prevents injuries, and that’s not something to gamble with.
Training Never Stops
Safety rules only work if people know and remember them—on tough days or when things get rushed. In my experience, the best-run shops run regular drills. Practicing how to put on gear, check meters, or clear the work zone creates habits that stick. Teams talk through what’s gone right and what needs to improve. Experienced workers take new folks under their wing so nobody’s left guessing. Updates come along every time guidelines change, or after any incident, big or small.
Better Engineering, Fewer Problems
NT system designers have a huge influence on safety outcomes. Good ventilation, double-wall piping, and pressure relief systems keep things safer from the start. If a plant manager puts money into maintaining equipment and upgrading valves, accidents drop. Automatic shut-offs and remote monitoring take workers out of the danger zone. Upgrades might seem costly, but the price of patching up major injuries, lost time, and lawsuits dwarfs them.
Sticking to the Basics
No shortcut replaces old-fashioned preparation. Reading the latest material safety data sheets, labeling containers right, locking valves during repairs, and refusing to take unexplained shortcuts all mean everyone clocks out in one piece. Asking questions and reporting odd smells or hissing noises keeps risks in check. It’s not about paranoia—it’s about respect for chemicals that don’t care what shift you’re working or how long you’ve been doing your job. Paying attention each time makes a real difference.
Getting to Know Chlorotrifluoroethylene
Chlorotrifluoroethylene—CTFE for short—shows up in places many don’t expect. This colorless gas has a faintly sweet odor and a chemical structure that packs three fluorine atoms and one chlorine atom around a pair of carbon atoms. That may not mean much standing alone, but those small details spark big changes in its behavior.
What Sets It Apart Physically
Working with CTFE feels different right away, especially once you see it escape from a cylinder. It’s a gas at room temperature with a boiling point near -28°C. That puts it below freezing for most of us, and it takes a pressurized tank to keep it in liquid form for handling or shipping. I’ve seen researchers get ready for CTFE use with heavy gloves—not because it’s caustic, but due to its chill.
CTFE is denser than air, so leaks tend to hug the floor or sink in confined spaces. Absence of a strong odor means you can’t trust your nose for safety. That always stuck with me as a reminder to check for proper ventilation.
Chemical Temperament of CTFE
The sharpest part of CTFE’s chemistry surfaces in how stable it acts. The bond between the carbon and fluorine atoms delivers remarkable resistance to heat, light, and chemical attack. CTFE won’t just break down under sunlight the way many other gases do. This sturdiness makes it attractive for making special polymers.
Mixing CTFE with other chemicals rarely sparks trouble at room temperature, but it does burn with difficulty in an open flame. The smoke releases toxic fumes, including hydrogen fluoride and phosgene. That’s a real risk. Lab tests show CTFE resists most common acids and alkalis, setting it apart for work with harsh substances or environments. My time around fluoropolymer labs made me appreciate that stability because cleanup after reactions becomes a whole lot easier.
Everyday Uses and Why They Matter
CTFE stands at the center of several modern plastics, especially polychlorotrifluoroethylene (PCTFE). These materials play a big role in aerospace coatings, chemical storage, and insulating films. Whenever I hear about a spacecraft or a pharmaceutical blister pack, odds are some variety of fluoropolymer built from CTFE lays beneath the surface. CTFE-based plastics won’t absorb water and they keep their shape across wild temperature swings, making them trustworthy where a breakdown would get expensive or put safety at risk.
Risks and Managing Them
Safety around CTFE starts with respect for what the gas can do and what it hides. Inhaling high concentrations can sicken or irritate lungs. Containers must be carefully checked for leaks—not just for health but fire risk too. I learned not to take shortcuts on ventilation or monitoring, because CTFE doesn’t give many warning signs before trouble. Still, with attention to basic lab protocols—leak checks, proper storage, and emergency plans—most problems stay firmly at bay.
For the environment, CTFE remains mostly contained in industrial cycles with limited direct release. Most regulatory agencies treat fluorines like CTFE with strict scrutiny, tracking emissions and setting limitations to defend water and air quality. For companies, adopting strong training and regular hazard reviews locks in those benefits.
Keeping Perspective
CTFE offers a sharp reminder: Small changes on the molecular level ripple out through the entire world of science and manufacturing. Knowing its properties inside out helps everyone—engineers, chemists, even health and safety teams—keep people safe while turning advanced polymers from theory to reality.
Understanding Chlorotrifluoroethylene
Chlorotrifluoroethylene, a colorless and flammable gas, finds a place in the chemical industry through its role in polymer and elastomer production. It doesn’t take an expert to know not all gases play nice with their environment. Years of experience in handling industrial chemicals have shown me: gas storage isn’t just about piling cylinders in a closet. Even the slightest oversight can bring risks for people, property, and the planet.
What Can Go Wrong?
Anyone who’s worked around reactive gases knows pressure and temperature shifts spell trouble. Most years in this field, I’ve heard of accidents tied to leaky valves or rusted tanks—almost always, someone figured: “This won’t happen to us.” Chlorotrifluoroethylene offers little forgiveness. If it leaks and meets a spark or open flame, explosions can tear through facilities. This gas forms toxic compounds in the air and reacts strongly with alkali metals, oxidizers, and even sunlight. If containment fails, workers could face serious health effects, including central nervous system problems and lung damage.
Responsible Storage: Facts, Not Guesswork
Safe storage doesn’t come by cutting corners. Gas needs a clean, dry, and cool spot, away from ignition sources. I always check for temperature swings because heat raises the pressure inside cylinders. With this compound, proper ventilation is non-negotiable. No one wants to breathe stray vapors. Containers must sit upright and get secured to avoid knocks that could shear a valve clean off, causing instant leaks. Metal cylinders store this gas best—no plastics, since Chlorotrifluoroethylene eats away at many synthetic materials.
Labels should stay sharp and clear. Confusion between cylinders has started more than one crisis. Flammable gas signs belong front and center, not hidden behind stacks of supplies. It pays to review the condition of every storage cylinder on a regular schedule. Rust, dents, or any odd smells should raise red flags immediately.
Backed by Science
Industry guidelines and scientific studies back up these steps. The National Fire Protection Association says to separate flammable gases from oxidizers and incompatible chemicals—a simple rule that gets skipped too often. The U.S. Occupational Safety and Health Administration (OSHA) requires storing compressed gases in ventilated areas, with strong supports in place. Their reports on chemical incidents show one recurring lesson: strong policy, backed by daily habit, saves lives and protects jobs.
Confidence Through Training and Transparency
Years of working with hazardous chemicals have left no doubt: training beats luck every time. Everyone on the floor needs to recognize what Chlorotrifluoroethylene looks and smells like, plus how to seal a leak and where emergency shutoff valves hide. No one should feel embarrassed to ask questions or double-check the process. In my experience, an open-door approach encourages quick reporting of even minor spills—sometimes stopping a headline disaster before it starts.
Insurance, audits, and regular emergency drills help keep facilities honest. Safety isn’t a single day’s work; it grows out of routine, shared knowledge, and a little healthy respect for what could go wrong. Companies that stay transparent about their storage practices build trust with workers and the wider community. None of this is glamorous, but it’s honest work—and it keeps lives whole.
Understanding What Chlorotrifluoroethylene Brings
Chlorotrifluoroethylene, or CTFE, turns up in more places than you might expect. Makers of specialty plastics count on it when making hoses and wiring for the aviation industry. Other manufacturers draw on its chemical properties for films and coatings meant to stand up to harsh chemicals. I once worked with folks in industrial settings where CTFE was handled, and back then not everyone stopped to question its safety. That comes with a cost, both for people and the world we live in.
Human Health Questions Keep Coming Up
People worry about more than just burns or spills. CTFE evaporates easily when heated, and those vapors don't just disappear. The Environmental Protection Agency lists this chemical as hazardous: breathing it, even in small amounts for a short period, causes irritation to lungs and eyes. Workers in older manufacturing plants told me stories about headaches, dizziness, and raw throats on the job, which lines up with published health data. That's not unique—plenty of halogenated chemicals trigger similar effects—but the risk becomes real in workplaces that cut corners.
The long-term health story isn't well mapped. Not every research group agrees on the cancer risk, but animal studies point to possible liver and kidney trouble if exposure runs high enough, for long enough. Cancer, reproductive problems, nervous system effects—these remain open questions. The chemical doesn't just slide through the body unnoticed; organs hold onto it for a while, buying more time for something to go wrong.
Environmental Effects Raise Red Flags
People sometimes forget about drift—what goes up in smoke usually comes back down somewhere else. CTFE doesn't stick around in soil or water like some other chemicals, but its breakdown products include toxic compounds, some of them even more persistent. Dumping or accidental release often means wildlife pays the price. Fish and smaller life forms up the food chain can't process chemicals like humans do, so a big enough leak spells trouble for them.
The ozone layer isn't a fan of chlorofluorocarbons, and CTFE carries that risk along with it. Some breakdown products poke holes in that crucial shield, so this isn't just a local hazard—it's the kind of thing that matters to everyone who relies on sunlight without worrying about skin cancer.
Safer Solutions and Smarter Handling
A few decades back, safety gear meant a dust mask and wishful thinking. We've moved beyond that, but not far enough. The companies staying up-to-date train workers on how to handle CTFE, invest in real ventilation systems, and push suppliers to cut emissions. That doesn't fix every problem, but it beats letting workers and neighbors take the hit. Enforcement stands on strong inspection and real penalties, not just paper rules.
The hunt for less hazardous alternatives matters most. Green chemistry, that push for useful stuff that breaks down harmlessly, deserves more attention. Smaller labs already test options that use fewer toxic inputs for similar performance. Sometimes, spending a little more up front drops the long-term cost to society and the planet.
Final Thoughts
People deserve honesty about what goes into the products they use and the air they breathe. Industry, regulators, and communities share responsibility for demanding more information and better safeguards. We don't always get to choose which risks show up in daily life, but turning a blind eye never did anyone a favor.