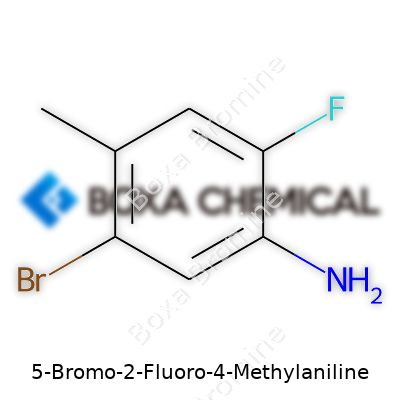
5-Bromo-2-Fluoro-4-Methylaniline: Detailed Commentary
Historical Development
Chemists have always pushed the boundaries with aromatic amines, and 5-Bromo-2-Fluoro-4-Methylaniline stands as one product shaped by decades of research in halogenated aromatics. As the pharmaceutical and agrochemical industries expanded, demand for specialized building blocks drove companies and academic labs to experiment with new synthetic pathways. The structure of this compound, featuring a bromine and fluorine on the aromatic ring along with a methyl group, emerged through detailed work in selective halogenation and functional group manipulation. The late twentieth century saw suppliers catalog such compounds more regularly, leading to higher purity grades and improved scalability for industrial needs.
Product Overview
5-Bromo-2-Fluoro-4-Methylaniline attracts attention because its motif slots easily into complex organic frameworks used in drug design. Lab workers recognize it as a valuable intermediate that supports modifications at the bromine and amino positions. Since fluorinated and brominated aromatics bring unique reactivity and influence pharmacological behaviors, companies package this substance in grades suitable for chemical synthesis rather than direct application in consumer products.
Physical & Chemical Properties
In hands-on experience, this compound appears as a solid—often a beige or off-white crystalline powder—with a distinct chemical odor shared by other halogenated anilines. The presence of both bromine and fluorine atoms raises its molecular weight and changes its solubility profile compared to simpler anilines. It dissolves fairly well in polar organic solvents such as DMSO or DMF, but less so in water. Its melting point lands higher than room temperature, making it easier to store and handle outside of specialized equipment. The compound’s aromatic ring changes electron density in significant ways due to the interplay between the electron-withdrawing and donating groups, which becomes crucial in synthetic planning.
Technical Specifications & Labeling
Every reputable supplier provides a clear certificate of analysis. Typical specifications highlight purity (often above 97% by HPLC), melting point range, and identification by proton NMR, IR, and mass spectrometry data. Labels carry CAS number (1190319-94-4), UN numbers if required, and standard hazard pictograms for handling. Batch numbers guarantee traceability, and packaging varies to minimize exposure—small glass bottles with Teflon-lined caps serve well for most laboratory scales.
Preparation Method
Synthesis begins with selection of the appropriate substituted aniline or halogenated toluene precursor. A common approach uses electrophilic aromatic substitution, adding bromine in presence of activating groups and controlling conditions to favor the 5-position. Next, selective fluorination requires methods that prevent overreaction or unwanted isomers, often guided by temperature and solvent choice. Crude product undergoes multiple purification steps, including recrystallization and column chromatography, to remove impurities and get a colorless, pure product suitable for sensitive downstream chemistry.
Chemical Reactions & Modifications
With its aromatic amine and halogen substituents, this compound participates in a diverse set of transformations. Classic examples include Buchwald-Hartwig amination at the bromo position to link other aromatic or aliphatic amines, or Suzuki-Miyaura couplings bringing in further functional diversity at the bromine site. The amino group itself lends to acylation, sulfonation, or diazotization, supporting synthesis of dyes, ligands, and pharmaceutical cores. Its fluorine atom can remain unaltered for medicinal chemistry, often helping increase bioavailability or altering metabolic profiles. Over time, researchers find new sites or methods for modifications, tracking results with precise analytical techniques.
Synonyms & Product Names
Product catalogs sometimes list this compound under alternative names such as “4-Methyl-5-bromo-2-fluoroaniline” or “2-Fluoro-4-methyl-5-bromoaniline.” Recognizing synonyms keeps procurement straightforward and avoids costly mix-ups. The CAS number stays the definitive identifier amid nationally or language-specific variations.
Safety & Operational Standards
Every handler must respect its classification as a potentially harmful aromatic amine. Solid lab routines require gloves, eye protection, and work inside well-maintained fume hoods to avoid skin contact and inhalation of dust or vapors. Material Safety Data Sheets point to risks such as skin sensitization, respiratory irritation, and possible environmental hazards due to halogen content. Proper waste collection and disposal—preferably by incineration—remains a non-negotiable. Companies selling and shipping this product follow strict labeling, transit regulations, and often offer technical support to help users meet workplace health and safety standards.
Application Area
Pharmaceutical researchers and agrochemical developers rely on this compound as a scaffold in synthesizing candidate molecules. Its well-placed halogens and amino group help explore structure-activity relationships when optimizing substances for anti-infective, anti-inflammatory, or herbicidal effects. Some material scientists turn to it for making specialty polymers or organic semiconductors, but the main market stays rooted in custom synthesis projects in medicinal chemistry labs and process chemistry scale-ups.
Research & Development
Laboratories keep pushing for more efficient, greener routes to make 5-Bromo-2-Fluoro-4-Methylaniline, seeking catalysts that work at lower temperatures or strategies leading to fewer side products. Analytical teams run advanced NMR, LC-MS, and high-throughput experiments to support QA and push discoveries about the compound’s behavior in new synthetic settings. Sometimes, published work inspires secondary applications, as patent filings indicate growing commercial interest and spurs fresh studies aiming to improve yields or downstream compatibility.
Toxicity Research
Unlike common industrial chemicals, detailed long-term human data remain limited for many fine chemical intermediates like this one. Animal tests and cell culture studies hint at risk pathways common to aromatic amines—skin and eye irritation, possible effects on blood health, and potential mutagenicity—so responsible scientists call for respect in all stages of use. Increasingly, regulatory agencies and university partners invest in new screening models, contribute toxicity data to public platforms, and help shape limits for workplace exposure and effluent discharge to protect both workers and surrounding communities.
Future Prospects
Trends in chemical synthesis lean toward more selective halogenation and the adoption of sustainable chemistry practices. 5-Bromo-2-Fluoro-4-Methylaniline fits these shifting priorities since its unique structure continues to attract attention from computational chemists modeling new drugs and from engineers scaling up pharmaceutical manufacturing. As green solvents and continuous-flow methods gain ground, researchers expect new preparation and modification techniques to match rising expectations for efficiency and environmental responsibility. Supply chain leaders work closely with regulatory bodies to ensure traceability, safety, and rapid response to any incidents or market changes, all while looking for new market niches and innovative uses for this well-designed building block.
Why Purity Matters When Working With Specialty Chemicals
Purity isn’t just a checkbox for chemical manufacturers. Small changes in the quality of materials can cost researchers valuable time. Take 5-Bromo-2-Fluoro-4-Methylaniline—an intermediate that gets a lot of interest in the pharmaceutical and agrochemical labs. Having spent time on both the campus bench and the industry floor, I’ve seen what happens when expectations around purity get overlooked: reactions fail, data turns unreliable, and projects run into endless troubleshooting cycles. Resources disappear into repeat experiments, eating up funding and testing patience.
The Actual Purity Numbers
The usual commercial purity for 5-Bromo-2-Fluoro-4-Methylaniline floats between 97% and 99%. This number isn’t pulled from thin air; suppliers use techniques like high-performance liquid chromatography and nuclear magnetic resonance. Reputable labs provide analysis so you see impurity profiles, not just a headline number. Tiny deviations—those two or three percentage points—tell a big story if you’re coupling or substituting this piece onto a larger molecule. The impurities can react, create byproducts, and sometimes skew results so badly that isolation or purification at the end of a multi-step process turns into a headache.
Quality Control and Its Impact
Over the years, the biggest headaches always began with overlooked quality. Somebody trusted an off-the-shelf order instead of checking the batch’s certificate of analysis. Out in real-world practice, high purity means reproducibility. You can predict yield, avoid mystery peaks in LC-MS analyses, and steer clear of safety surprises that pop up from unknown contaminants. Buying from vendors who perform and publish full characterization—NMR, IR, melting point, even residual solvents—always pays off in saved time and clean data.
Facts From Regulated Fields
The market for custom synthesized intermediates, like 5-Bromo-2-Fluoro-4-Methylaniline, is expected to keep growing. According to industry studies, pharma companies spend billions annually on quality control for raw materials. Their rules—according to the International Council for Harmonisation and local authorities—require traceability in purity and composition. If contamination shows up in anything headed for drug development, projects can be suspended during critical stages. Regulators don’t just want to see percentages—they expect full impurity profiles, which gets expensive fast if the starting materials cut corners.
Responsible Sourcing—A Personal Perspective
Experience taught me to vet every new supplier before ordering a specialty compound. The biggest red flags come from companies that dodge questions about origin or refuse to show batch analytics. Reliable partners openly discuss storage, transport, and analysis methods. They respond to technical questions without hesitation. Transparency sets trustworthy suppliers apart, especially when sourcing chemicals that can affect outcomes and safety.
Moving Toward Better Practices
Investing in top-quality chemicals begins in the planning stage, not after a synthesis goes sideways. Teams can insist on seeing current batch analytics, run in-house quality checks, and establish long-term relationships with vendors whose credentials stand up to scrutiny. Open communication and sharing best practices across organizations makes a difference. I’ve seen collaborative groups catch purity issues early, saving months of downstream cleanup.
Solutions That Put Science First
Checking purity goes beyond looking for a big number on a datasheet. Teams ensuring every batch of 5-Bromo-2-Fluoro-4-Methylaniline has been tested for known and unknown impurities actually end up spending less time in damage control. Real investment in reliable analysis—along with full traceability—brings peace of mind and cleaner results.
Understanding the Everyday Realities of Chemical Storage
Most chemists who spend time in the lab will recognize the value of setting up safe, sensible storage for chemicals that don’t see the light of day very often. 5-Bromo-2-Fluoro-4-Methylaniline belongs to a family of aromatic amines where a small slip, like keeping the bottle in the wrong spot, can create headaches later on or, much worse, put someone at risk. Over the years, I’ve seen solvents spoil and reagents break down—wasted money, wasted time, and sometimes dangerous surprises. You learn quickly that following basic rules can make all the difference.
Shielding the Compound from Environmental Factors
Aromatic amines such as 5-Bromo-2-Fluoro-4-Methylaniline tend to degrade in the presence of air, heat, and light. My own experience tells me that controlled temperature and shielding from sunlight prevent problems like slow decomposition and changes in physical appearance. Storing it in a cool, dry location out of direct light preserves the material’s intended structure. A refrigerator set to 2–8°C usually works well, although not every benchtop refrigerator is equal. Dedicated chemical refrigerators, with clear segregation between solvents and perishable substances, help reduce risks of cross-contamination.
Keeping Moisture at Bay
I’ve lost more than one vial of specialty chemicals to humidity sneaking in. For compounds like 5-Bromo-2-Fluoro-4-Methylaniline, moisture may not be catastrophic, but it can bring slow hydrolysis or clumping, especially after repeated opening of the container. The simplest fix: toss in a silica gel packet before sealing the bottle. Silica works without fuss and costs almost nothing. Tight, screw-cap vials made from glass with PTFE liners keep environmental exposure to a minimum. Some labs use desiccators for extra confidence—especially if the chemical doesn’t see regular rotation.
Clear Labelling and Documentation
More than once, I’ve run into bottles with faded permanent marker or half-peeled labels. These mysteries waste hours and stall projects. Clear labelling, recording the date received and date opened, gives everyone on the team a quick overview. Lot numbers and supplier information can be critical if a batch later comes under question. Organized records keep the lab running and support essential traceability, a major point in regulatory audits and overall trustworthiness.
Minimizing Exposure, Handling with Care
Even stable compounds can go off if left open on a bench during a day of trial and error. Returning the bottle to storage right after each use keeps its integrity intact and protects anyone passing by. Disposable nitrile gloves, safety goggles, and fume hoods—these aren’t status symbols but practical gear that protect everyone’s health. A fume hood provides extra insurance, especially with aromatic amines, because even small amounts volatilize faster than you’d expect at room temperature. I’ve seen bottles set to the side as “temporary,” only to discover leaks or odors within hours.
Building Better Habits for the Whole Team
Every lab can set up a system where colleagues remind each other about proper storage. It isn’t about being a stickler; it’s about avoiding emergency calls in the middle of the night or wasted budgets at the end of a funding cycle. Refresher sessions and clear signage go a long way. A culture where every person values storage discipline pays off in reliability and trust. The best chemical storage shelf isn’t hidden away—it’s a central part of safe science every day.
Organic Synthesis Kicks Things Off
5-Bromo-2-Fluoro-4-Methylaniline has turned into a bit of a secret ingredient for chemists who spend their days building new molecules. That bromo and fluoro flavor means researchers can use it as a building block in a ton of different ways. Want to build a pharmaceutical intermediate or craft an agrochemical? This compound gives you a start thanks to that reactive bromine and also the nuanced effects from the fluorine atom.
Pharmaceutical Research Relies on This Toolbox
Modern drug discovery feels like fishing sometimes, trying out different lures until you land on something a patient might benefit from. Medicinal chemists often reach for aromatic amines like this one when they’re fishing for new compounds. That is because adding both bromine and fluorine atoms onto a benzene ring cranks up the chances that a molecule will behave differently inside the body. It might make a compound last longer in your bloodstream or help it fit into a disease target a little tighter. I’ve seen projects where a swap from methyl to fluoro or bromo flipped a dead-end idea into an early candidate. It happens more often than you’d think.
Agrochemical Discovery Keeps Growing
Finding new protection for crops means testing a wild range of chemicals. Compounds with these halogen atoms tend to resist breakdown from sunlight and plant enzymes, helping any new pesticide stick around long enough to do its job. Agrochemical formulation teams often look for ways to keep breakdown slow but safe, so a compound like this gives researchers a springboard for new ideas. I’ve talked with folks who have walked dozens of similar compounds through field trials, only to see one with a bromine atom punch above its weight.
Advanced Materials and Dyes: Coloring Outside the Lines
If you mention “aniline” to anyone in the colorant or plastics world, their eyes light up. A few tweaks on the basic aniline structure let companies build everything from heat-resistant polymers to next-generation dyes. Drop a bromine on one side and a fluorine on the other, and suddenly you’ve got options for changing a dye’s shade or making a plastic sheet withstand more abuse in the sun. Material scientists seem to love playing around with these kinds of tweaks. The neat thing is how one small change can make a pigment bolder or a coating last a few more seasons on an outdoor billboard.
Keeping Safety and Stewardship Front of Mind
With all these uses, safety stories run alongside every experiment and batch. Compounds with both fluorine and bromine can stick around in the environment, so the responsible path means strong containment, careful waste handling, and following all the modern guidelines for synthetic chemicals. I’ve watched some of the best chemists I know design new routes that reduce leftover halogenated waste. Green chemistry isn’t just idealism—it’s becoming the standard, especially as companies and universities relate to bigger public concerns about persistent chemicals.
Science Moves Forward With Sharing
Behind every bottle of 5-Bromo-2-Fluoro-4-Methylaniline sits a notebook full of hard-won knowledge. Whether it turns up in anti-cancer research, pest management, or the chase for a brighter yellow pigment, its role keeps evolving. The more openly we share discoveries and lessons, the better the outcomes for public health, the environment, and those future scientists who will find new ways to make this molecule work for all of us.
Understanding CAS Numbers: Why Bother?
Standing inside a bustling chemistry lab, surrounded by shelves crammed with bottles marked by codes and symbols, it felt overwhelming. One morning, hunting for a rare aromatic amine, I realized how much confusion arises without a simple identifier. The Chemical Abstracts Service (CAS) number, a unique numeric label for every chemical substance, changes the game. For 5-Bromo-2-Fluoro-4-Methylaniline, the CAS number is 57381-16-7. Try sharing a molecule’s full name during a heated team discussion—somebody always hears it wrong, scribbles the formula off by a letter, or mistakes it for a similar compound. With a CAS number, the mess stops.
Keeping Safety Standards High
Lab accidents often hide behind something as simple as naming confusion. Early in my career, someone mislabeled a benzenoid compound. The repercussions weren’t mild. The wrong chemical ended up in a reaction that released fumes and forced an evacuation. With strict reliance on a CAS number, such mistakes drop dramatically. Safety data sheets, regulatory paperwork, and even import-export forms rely on that little string. The number 57381-16-7 carries the entire molecular fingerprint of 5-Bromo-2-Fluoro-4-Methylaniline across the globe—and no language barrier messes with it.
Research, Patents, and Product Quality
In research, hunting down the right articles or patents gets much faster with correct identification. During a project digging into new dye intermediates, my team hit a snag because a single paper used an archaic name. Searching by CAS number swept in a trove of relevant data we’d otherwise have missed. Patents hinge on ironclad definitions. Without this kind of precision, intellectual property slips through loopholes. A molecule as niche as 5-Bromo-2-Fluoro-4-Methylaniline falls under multiple names but just one CAS number so inventions stay protected.
Quality Control in Manufacturing
Anyone who’s worked in chemical production knows the headaches caused by receiving the wrong raw material. A small difference in molecular structure can mean wasted batches, regulatory hassles, and battered trust with customers. Specifying the correct CAS number streamlines procurement and inventory. Suppliers around the world recognize 57381-16-7 the same way, slashing miscommunication and costly returns. This accuracy shows up in everything from new pharmaceuticals to better electronics coatings.
Global Regulations and Human Safety
Regulatory authorities in different countries lean on CAS numbers to synchronize chemical safety. The United States Environmental Protection Agency, the European Chemicals Agency, and customs officials everywhere use these numbers. This keeps dangerous or restricted substances from sneaking across borders under vague names. For a compound like 5-Bromo-2-Fluoro-4-Methylaniline, environmental specialists and toxicologists use the CAS to pull toxicity profiles, exposure limits, and disposal protocols without wading through ambiguities.
Building Trust in the Chemical Supply Chain
Trust grows when everyone speaks the same language. In the chemical sector, that language is often numeric: the CAS number. Whether you’re a researcher, a factory manager, or an importer, seeing 57381-16-7 on the bottle removes guesswork. Strong communication, fewer errors, and safer outcomes all trace back to a simple, reliable identifier.
Spotlight on Lab Safety
Anyone who’s worked in a chemistry lab knows the drill: you open a fresh bottle of a strange compound, the scent hits, and you hope someone bothered to store the Safety Data Sheet nearby. For 5-Bromo-2-Fluoro-4-Methylaniline, curiosity runs high because you probably won’t find its SDS stapled to a bulletin board in most workplaces. This compound lands in a family of substituted anilines, which carry plenty of red flags. A datasheet isn’t just a bunch of bureaucratic nonsense — it can be the difference between a safe experiment and an ambulance call.
Digging for the Details
It’s always surprising how fast the need for clear, transparent chemical safety information shows itself. Even for a compound like 5-Bromo-2-Fluoro-4-Methylaniline — not exactly a household name — the right data sheet cuts through confusion. Without one, you guess about handling hazards, potential for skin or respiratory irritation, fire risks, and proper storage or disposal. Some suppliers do make these sheets available, like Sigma-Aldrich and Alfa Aesar. Still, plenty of times you chase a chemical’s SDS only to find generic versions or files in a foreign language.
The Reality in Research and Industry
Working with aromatic amines can get risky. Simple protocols — gloves, goggles, good ventilation — become non-negotiable habits. An SDS lays out the roadmap for what to do when things go wrong: eye contact, accidental spillage, a sudden exposure. Years ago, I watched a colleague pop the stopper on another aniline derivative, get a faint mist, and cough for days after, even with a hood running. That sheet, right next to the hood, spelled out what he should do, which let everyone act fast.
Beyond Compliance — Practical Solutions
Regulatory agencies like OSHA and REACH make calls on SDS availability, but enforcement doesn’t always trickle down everywhere. Chemists shouldn’t wait for someone else to download the SDS or print it out. Companies need to keep digital and physical copies on hand and make it part of laboratory onboarding. Researchers and staff can spend a few minutes reading the hazards, first aid, and firefighting measures. The habit sticks after one incident turns messy.
As labs get busier, shortcuts happen. Someone might use an unlabeled bottle assuming it’s benign, or toss waste where it does the most harm. That’s when the SDS becomes more than a legal requirement. It feels personal when mistakes hit close to home.
Empowering Safer Practices
Access to an accurate SDS for specialized compounds like 5-Bromo-2-Fluoro-4-Methylaniline creates a baseline of trust between chemists and their environment. There’s no harm in over-preparing. Team briefings, up-to-date documentation, and easy access to the latest data must become part of the workflow, not an afterthought.
Knowledge, after all, means fewer nasty surprises. As more specialty chemicals enter research spaces, those first few minutes spent with a safety sheet can make all the difference in keeping every set of hands steady and safe.