2-(2-Chloroethoxy)Ethanol: A Real-World Perspective
Historical Development
Scientists started paying attention to 2-(2-Chloroethoxy)ethanol decades ago, around the mid-20th century. The chemical industry saw promise in modifying smaller glycols to fit new tasks cropping up—polymer science, solvent technology, or chemical synthesis work. Interest grew as laboratories found ways to attach the functional chloro group to a chain already bearing a hydroxyl group, chasing both reactivity and new downstream products. Soon after, patents sprang up describing routes that saved time or raw material. Experienced chemists recall the persistent push for quality controls by the 1970s, aiming to minimize contaminant levels and unwanted isomers. Today, the compound’s legacy traces the arc of wider industrial growth where improving purity and process safety kept pace with demand in labs and plants alike.
Product Overview
Manufacturers produce 2-(2-Chloroethoxy)ethanol as a colorless or pale yellow liquid. It runs with a slight, often sweetish odor, easily picked up by an experienced technician. Chemists prize it for its dual functional groups—both a reactive chloro and a hydroxyl—put there to support downstream chemistry. Businesses ship the product in drums or smaller glass containers, depending on scale, and each batch enters a growing registry for chemical exports and imports. Over the years, the turnover numbers climbed, especially for producers in Europe and Asia, reflecting a steady pull from downstream intermediates, especially when tighter quality checks entered the mainstream.
Physical & Chemical Properties
Measured by density, 2-(2-Chloroethoxy)ethanol falls in the mid-range compared to other glycol derivatives. It mixes with water but doesn’t dissolve everything as completely as pure glycols do—solubility numbers depend on temperature and solution makeup. It boils above 200°C, while freezing occurs far below room temperature, keeping it liquid in most settings. Chemically, the chlorine atom on the ethoxy side opens options: nucleophiles can displace it, setting off a cascade of potential reactions. Over time, folks working with the compound learned its reactivity follows trends they know well: the presence of both hydroxyl and chloride gives rise to esters, ethers, and more, provided reaction partners suit the task.
Technical Specifications & Labeling
Producers standardize this compound at high purities—often exceeding 98%—and define water and byproduct limits tightly. Spec sheets, based on years of lab and regulatory experience, always list appearance, assay percentage, water content by Karl Fischer titration, color by Hazen units, and a profile of known byproducts. Containers carry clear hazard pictograms: the GHS07 exclamation mark warns users about irritation risk, and companies translate hazard phrases alongside batch numbers, gross weight, net content, and supplier traceability. Storage instructions favor cool, dry areas away from acids, bases, and strong oxidizers, with plenty of companies moving to tamper-evident lids or seals after a few high-profile supply chain scares.
Preparation Method
Making 2-(2-Chloroethoxy)ethanol generally starts with ethylene glycol, which reacts with an appropriate chlorinating agent like ethylene oxide and hydrogen chloride—or through more direct alkoxylation and subsequent chlorination. Most producers favor methods that reduce side-products, so continuous reactors and rigorous washing steps rule the day. Lab veterans recall the days of glass columns, brisk water scrubs, and careful distillation to hit targets for clarity and odor. Now, process engineers fit solvent recovery and vent treatment technology onto commercial runs, partly in response to stricter air and water regulations.
Chemical Reactions & Modifications
This compound’s uses rest on its lively reactivity. The chloride on the molecule beckons for nucleophilic substitutions—classic SN2 chemistry. Pair it with an amine, and you’ll swap that chloride for a nitrogen, opening the route to tailored amines. The hydroxyl group, too, never sits idle: chemists esterify it to produce specialty solvents, pharmaceuticals, or plasticizers. Both industrial chemists and academic researchers explore these functionalizations, competing to find creative uses in fine chemicals. The molecule’s backbone even serves as a short-chain spacer in complex macromolecules. Modifications often target applications in drug intermediates, dyes, or crosslinking agents, making the compound valuable for more than just its starting structure.
Synonyms & Product Names
You’ll find 2-(2-Chloroethoxy)ethanol listed as Chloroethoxyethanol, Ethanol, 2-(2-chloroethoxy)-, or simply CEE in chemical directories. Substituted glycol derivatives share similar naming conventions and can trip up less experienced hands at the bench, but CAS number cross-checks keep clarity high. Commercial suppliers market the chemical under a variety of trade names, sometimes with slight tweaks to the nomenclature. For laboratory supply, labels often include both systematic and common forms, depending on the practice of the company.
Safety & Operational Standards
Working with this compound demands care. It can irritate eyes, skin, and respiratory tract—sometimes giving delayed reactions. Gloves, goggles, and fume hoods usually show up on shop floors and research benches alike. Experienced workers take these hazards seriously, pushing for material safety data sheets and refresher training as turnover brings in new hands. Emergency protocols for spills lean on neutral absorbents, careful disposal, and minimum exposure, always updated in light of evolving best practice. In some countries, regulators now set workplace exposure limits and require continuous air monitoring, thanks to prior lessons on chronic effects and worker compensation claims.
Application Area
Paint labs and resin formulators saw value early, using 2-(2-Chloroethoxy)ethanol as a reactive intermediate in synthetic resins, plasticizers, and surfactants. Its byproducts make appearances in pharmaceuticals and agrochemical synthesis, often as chain extenders or functional group placeholders. Over time, water treatment facilities and electronics manufacturers adopted related compounds for specialty tasks, thanks to the molecule’s controlled reactivity. On the research side, you’ll run into it wherever folks need a short, reactive chain that can blend into larger structures, from conditional-release drugs to cross-linked polymers and advanced lubricants.
Research & Development
Older journals highlight a lot of foundational work on new reaction paths and safer process options. In the last two decades, green chemistry initiatives have pressed for methods that limit waste and use milder reagents. Lab crews pivot to one-pot syntheses and solvent minimization, hoping to trim energy use and lower persistent contaminant loads. R&D teams in major chemical companies prioritize lifecycle assessments; they want not just reaction efficiency but also real gains in end-user safety. Machine learning models now help map predictive reactivity across related chloroalkyl alcohols, and several patent filings chase process optimizations or novel end-uses.
Toxicity Research
Toxicity remains a live topic, as both acute and chronic exposure risks grab institutional attention. Researchers track dermal, oral, and inhalation pathways: animal studies tell of liver and kidney strain at high doses, while cell assays show DNA and protein effects in select lines. Most risk comes down to workplace exposure, with reports of eye and skin irritation still cropping up. Longitudinal health studies have yet to pin down all chronic impacts, but regulatory filings now demand comprehensive hazard communication and robust workplace controls. Waste handlers especially push for clarity on breakdown products and persistence in water tables, since similar chemicals sometimes resist ordinary treatment plants. Regulatory agencies in Europe and North America revisit these reviews every time new test models or reports surface.
Future Prospects
Looking at the years ahead, the market for this compound ties directly to both regulatory oversight and demand from developing chemical sectors. As pharmaceutical and specialty polymer labs ramp up, requests for this kind of intermediate tick higher, provided greener and safer routes stay viable. Researchers keep searching for catalysts and processes that cut down byproducts and sharpen yields, both for cost and sustainability. Improvements in process safety—like better sensors, new containment tech, and more effective workplaces—should further reduce risks tied to acute and long-term exposure. If future studies clarify chronic risks, companies will need to adapt fast, possibly shifting toward derivatives that keep utility high but reduce hazard levels. Many in the field expect growth to continue, though at a pace shaped by both technical innovation and tightening health and environmental rules.
Hidden Workhorse Behind Everyday Products
Few outside laboratory walls recognize 2-(2-Chloroethoxy)ethanol. Its chemical name doesn’t roll off the tongue, yet this liquid quietly underpins a web of essential products. Found in industrial settings, it's not sold on supermarket shelves, but traces of its impact pop up in our lives more often than expected.
Role in Chemical Manufacturing
Chemical plants use this compound as a basic building block. It helps create other chemicals that end up in plastics, lubricants, or specialized cleaning solutions. Chemists value its two functional groups: an alcohol and a chloro group. This combination allows targeted reactions, helping link molecules in ways that open doors to a broader range of materials. Its chemical flexibility pushes the boundaries for scientists aiming to make new surfactants, solvents, and pheromone components.
What many don’t see: common materials—from the coatings on wires to the smooth finish on print inks—sometimes owe a tip of the hat to the presence of 2-(2-Chloroethoxy)ethanol in their birth process. Without intermediates like this, polymer factories would struggle to keep up with demanding product specs and tighter rules on energy use.
Not Just a Cog: Safety and Responsibility Matter
If you’ve ever spent time in an industrial lab, you’ll know how important handling protocols become with chemicals like this. The chlorine atom makes it active and valuable, but at the same time heightens the need for control: gloves, goggles, and fume hoods come standard. This isn’t a risk to everyday consumers—the compound rarely, if ever, shows up at home in its raw form—but carelessness at the factory step can have big consequences.
Regulatory rules rightly demand oversight. I’ve watched safety meetings focus on disposal strategies, since even small spills can cause trouble. Turns out, the same attributes that make this compound effective at changing molecular structures can also mean harm to health or waterways if released. Embracing closed system production, robust training, and enforced waste management minimizes those risks. Better tracking methods could help spot issues faster and keep workplaces safer.
Looking Forward: Sustainable Chemistry
Green chemistry trends are nudging all parts of the industry to get better at recycling intermediates like 2-(2-Chloroethoxy)ethanol or swapping them for cleaner alternatives where possible. Researchers watch toxicity data closely and push for options that lower emissions from beginning to end. New breakthroughs, such as biosynthesis alternatives or process tweaks, might one day cut chlorine use or make safer versions.
Even with sustainability goals in mind, demand for chemicals that deliver reliable reactions sticks around. My experience shows progress moves fastest when companies invest in continuous staff training, update their tech regularly, and work with regulatory agencies—not against them. This approach helps chemical intermediates like 2-(2-Chloroethoxy)ethanol continue serving their purpose without putting people or the planet on the line.
What Makes 2-(2-Chloroethoxy)Ethanol a Concern?
2-(2-Chloroethoxy)ethanol pops up in industrial settings, mostly in chemical manufacturing or as a solvent. Anyone working in an environment where this compound floats in the air or contacts your skin should pay attention. Here’s why: this chemical carries real risks that aren’t just paperwork—these risks can show up as symptoms on the job or long-term damage that’s hard to undo.
Lab data shows 2-(2-Chloroethoxy)ethanol can irritate skin and eyes quickly. The compound gets absorbed rapidly, so even a quick splash on bare skin delivers a dose right into your system. Over time, repeated contact ramps up the risk. Eye contact causes stinging and redness, reminding you in a hurry this isn’t something to brush off.
Long-Term Risks and Exposure Pathways
Workers breathing in vapors or getting the chemical on their skin face bigger dangers than immediate irritation. Research links this compound to organ toxicity. The liver and kidneys—the body’s filers—pick up a lot of the slack, so they’re at higher risk. A person may not spot trouble for a while, and that’s the scariest part. Lab studies show that liver enzymes in mammals rise with repeated exposure, suggesting damage builds up bit by bit.
There’s another layer here: 2-(2-Chloroethoxy)ethanol belongs to a family known for creating toxic byproducts. When not handled properly, these byproducts hang around in the environment, polluting water and soil. Even folks far from the original worksite might experience the side effects if runoff seeps into local groundwater.
Why Following Safety Rules Matters
Personal experience shapes my attitude toward chemical safety. A buddy of mine spent years in a plant and shrugged off gloves and goggles for convenience. After a few months, rashes started to show up. Hand-washing helped at first. Over time, problems grew. By the time bloodwork flagged issues, he had to leave the job for good. Simple steps—gloves, sleeves, sealed containers—might sound dull, but they’re proven to make the difference.
The facts say the same thing. OSHA and the European Chemicals Agency list 2-(2-Chloroethoxy)ethanol as a hazardous substance, recommending strict handling guidelines. Proper ventilation, eye protection, and chemical-resistant clothing are not just red tape. These rules shield workers from direct contact, inhalation, and splashes that turn small exposure into a big problem.
Moving Toward Safer Workplaces
Solutions start with education. People need real information about the chemicals they use every day. Training should cover more than the basics—it works better with personal stories, up-to-date research, and clear steps for handling spills or accidental splashes.
Safer chemicals exist for some applications, and it pays to swap them in where possible. Substitution cuts risk for everyone along the supply chain. Real enforcement of safety rules also matters. Management must back up safety procedures with the right gear and regular reminders, not just posters on the wall.
Communities living near facilities using 2-(2-Chloroethoxy)ethanol deserve transparency too. Honest communication builds trust and helps people spot and report environmental problems before they spread. People shouldn’t have to guess what’s getting into their water or air.
What Counts Moving Forward
2-(2-Chloroethoxy)ethanol brings specific hazards that nobody should ignore. Trusting luck or shortcuts leads to real harm. Scientists, workers, and local residents all have a stake in how industry handles this chemical. Prioritizing safety cuts down on illness today and keeps problems from getting bigger down the road.
Breaking Down the Structure
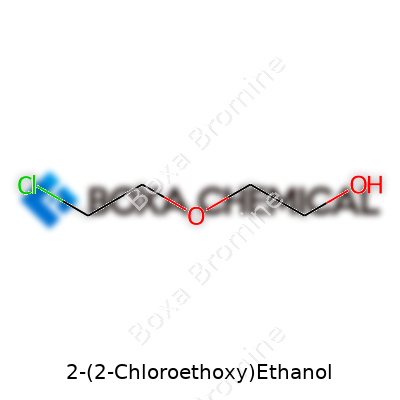
2-(2-Chloroethoxy)ethanol comes with a chemical formula of C4H9ClO2, which can also be outlined as ClCH2CH2OCH2CH2OH. This structure offers a lot more than what meets the eye. The molecule contains four carbon atoms, two oxygen atoms, and a single chlorine atom. Dive into a chemistry textbook and it won’t take long to realize the impact chlorine brings to ethoxy derivatives—it creates possibilities for new reactions, impacts solubility in water, and shapes how the compound interacts with living systems and industrial materials.
Why Chemical Structure Matters
Personal experience in a laboratory teaches that the detail isn’t just in the big flashy reactions. Tiny alterations—like swapping a hydrogen for a chlorine—change the game completely. That’s what’s happening with 2-(2-Chloroethoxy)ethanol. Its backbone looks a lot like standard diethylene glycol, but the chlorine gives it increased reactivity, letting scientists use it for more specialized syntheses in pharmaceuticals, pesticides, and even polymer precursors.
Real World Uses and Consequences
Industries pick this compound for reasons grounded in its chemical versatility. The solvent properties carry weight, especially when working with resins or formulating custom surfactants. Chemistry isn’t about memorizing formulas—it's about understanding what a small tweak brings. Chlorinated compounds show up in a lot of useful and hazardous places. This molecule could shape better drugs one day, or contribute to greener chemistry by making traditional syntheses easier or safer. On the flip side, the presence of chlorine means waste streams need to be managed with care; these molecules can become persistent environmental challenges if they leak outside lab walls.
Health and Safety Concerns
It’s easy to gloss over the dangers, but safety matters here. Chlorinated alcohols can be eye and skin irritants. Breathing in vapors doesn’t do anyone’s lungs a favor. Chemistry students tend to focus on results, but anyone who has handled a leaky bottle of this stuff knows how important it is to use fume hoods and gloves. Solvents don’t just evaporate and disappear—they can linger in the workspace, potentially affecting air quality.
Better Practices for Handling
From firsthand observations, smart handling cuts risk sharply. Secure storage, proper labeling, and use of spill containment all keep risks low. It pays to think about the route this compound takes once the experiment finishes. Waste treatment teams need to break down any residues, neutralize hazards, and keep potentially harmful derivatives out of rivers and soil. More research is pushing chemists to create similar compounds from renewable feedstocks, or to redesign recipes that minimize chlorine content without losing function.
Closing Thoughts on the Impact of C4H9ClO2
The formula for 2-(2-Chloroethoxy)ethanol looks simple enough, but every atom counts. From new product synthesis to lab and environmental safety, understanding the makeup of what sits on your bench gives real-world power. That’s where the future of science begins—by knowing the details and taking responsibility for them.
What Sets This Chemical Apart
2-(2-Chloroethoxy)Ethanol draws attention not because it’s flashy, but because a small mistake can mean headaches—or even danger. Used in labs and some industrial production, this chemical packs a punch in reactivity. Its structure includes an ether and a chlorine atom, which makes it both handy for synthesizing new molecules and risky in poorly controlled environments. Anyone who spends time around chemicals knows: the dull liquids are often the ones to take most seriously.
Why Storage Practice Matters
From my own days in research, I learned early that a clean, labeled shelf isn’t enough when you’ve got solvents or reagents with a chlorine group. Leaky lids, heat, or the wrong neighbor in the cabinet and your so-called routine project spirals into a call for the emergency response crew. 2-(2-Chloroethoxy)Ethanol evaporates more quickly than some water-based chemicals. Breathing in its vapors leads to dizziness, sore throat, or in rougher cases, damage to internal organs over repeated exposure. Even if you’re not handling gallons of it, a small spill in a cramped workspace means inhalation risk is real.
Getting Serious About Protecting People and Property
The right approach starts before the bottle even shows up. Only bring in what you’ll use soon. Extra containers sitting around act like forgotten eggs—sooner or later, one cracks. Good storage happens in a cool, well-ventilated, locked cabinet. No excuses. Keep it away from sunlight or any heat source, since both raise pressure inside bottles. And don’t ignore those hazard symbols. They mean something, especially on a bottle marked irritant or health hazard. I once saw a coworker grab the nearest empty glass jar for temporary storage—an innocent shortcut led to a melted label, sticky shelf, and a mystery spill nobody wanted to claim.
Separation and Compatibility
Leave it away from acids, bases, and oxidizers. Mixing even residual amounts can trigger chemical fires or release toxic fumes. At some older facilities, legacy storage still groups everything organic on the same shelf. That shortcut no longer cuts it. Create a practice where only compatible chemicals share a shelf. If the label has faded or the contents are unclear, treat everything as high risk until identified. Regularly check containers for swelling, cracks, or corrosion around lids. These are early warnings before real trouble hits.
Labeling, Records, and Eye Contact with Reality
In college, I failed to mark a bottle with a fresh date. Three months later, the clear liquid had picked up a yellow tint, and the lab instructor nearly blew a gasket. Every use and every transfer gets logged. If someone needs to find out what’s in the cabinet at three in the morning, they shouldn’t have to guess. Unlabeled, unknown chemicals have sparked too many close calls. The habit of double-checking changes in appearance or smell (without direct sniffing!) saves more than time—it saves lives.
Solutions for Smarter Storage
Modern labs rely on chemical tracking tools and safety trainings that actually walk through common scenarios. Automated reminders for expired or unused chemicals catch lapses before they snowball. At home or in smaller shops, clear plastic boxes inside vented cabinets block leaks from spreading. Personal protective equipment lies waiting next to chemical cabinets, not lost in a drawer. Routines get written down and practiced, not left to chance. Staff swaps out cracked lids on sight, not once a year during spring cleaning.
Learning from Close Calls
No one stays immune from complacency. Just last year, an experienced technician in a big-city research hospital ignored a cabinet temp sensor. By the time he noticed, pressure warped a container and forced an evacuation. Direct experience with mistakes—our own or others—teaches faster than any policy memo. Real safety culture starts with one decision at a time, every day. For 2-(2-Chloroethoxy)Ethanol, respect and routine are the best shields we’ve got.
Direct Contact and Inhalation Risks
Handling chemicals like 2-(2-Chloroethoxy)ethanol calls for respect and a careful approach. Skin absorbs this liquid quickly. Once that happens, the path to headache, dizziness, or even more severe symptoms opens up without much warning. Splashing chemicals onto uncovered hands is a shortcut to doctor visits, so basic gloves made from nitrile or rubber become your best friends in the lab or factory.
Eyes deserve equal attention. Accidents in tight spaces or crowded workshops tend to end with splashes or spills that go right into the face. Safety goggles, snug and clear, cut that risk to nearly zero. Rubbing your eyes with contaminated hands can cause long-lasting damage, so frequent handwashing can't slip down the priority list.
Breathing Hazards and Ventilation
Working with volatile chemicals means dealing with invisible dangers. 2-(2-Chloroethoxy)ethanol evaporates and sends small particles into the air, especially if the liquid gets warm. Inhaling those vapors doesn’t always hurt right away, but long-term exposure weighs heavy on both lungs and liver.
Open windows and basic fans don’t cut it in most labs. Mechanical ventilation, well-maintained and well-placed, keeps the air clear. Fume hoods, checked regularly, make a big difference during weighing and mixing. For jobs that push up the risk, properly fitted respirators turn out to be more than a recommendation—they save health and productivity.
Fire and Chemical Spill Dangers
2-(2-Chloroethoxy)ethanol burns easily when it meets a spark or overheated surface. That makes fire extinguishers, especially those rated for chemical fires, as important as any beaker or pipette. Sparks from equipment or static buildup from plastic containers—simple things cause big problems. Grounding and using explosion-proof switches gives peace of mind that no small spark will set off a blaze.
A spill doesn’t stay harmless on any shop or lab floor. Absorbent pads and chemical-resistant spill kits need to sit within arm’s reach. Wiping up with regular towels just spreads the risk to your hands and whatever else it touches. Disposal rules say to seal used pads in proper containers, keep them away from regular trash, and label everything so even the janitor knows what’s inside.
Personal Habits and Ongoing Training
No manual or sign replaces personal responsibility. Eating at your workbench or drinking coffee with chemical-stained gloves turns small mistakes into big emergencies. Taking off gloves before touching your phone or face, washing hands before meals, and storing work clothes separately from street clothes seem basic, but these habits quietly prevent weeks off from work.
Annual safety refreshers, quick quizzes, or real-life practice drills make safety rules stick. A practiced hand recognizes strange smells or headaches and knows when to speak up. Open chats about mistakes, not blaming or hiding them, often keep coworkers out of the local ER.
Safer Substitutes and Engineering Controls
Before reaching for 2-(2-Chloroethoxy)ethanol, a check for safer substitutes sometimes pays off. If a process lets you swap to a less toxic chemical, health improves and headaches from audits drop. For those times when only this compound gets the job done, strong storage rules, clear labeling, double-checking expiration dates, and keeping containers tightly sealed all keep accidents rare.
Safety depends on steady habits, good gear, and the willingness to step back and double-check before each shift. Protect yourself, coworkers, and the environment, one step at a time, every day.