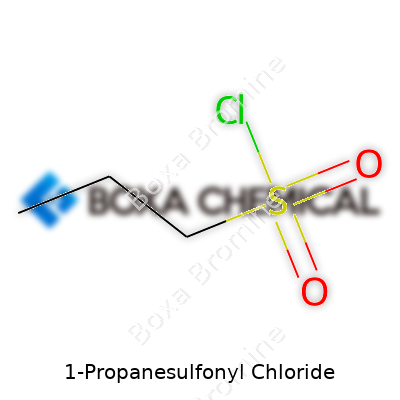
1-Propanesulfonyl Chloride: History, Properties, and Outlook
Historical Development
Chemistry has never stood still, and 1-Propanesulfonyl Chloride proves that point. Back in the mid-20th century, researchers explored new ways to introduce sulfonyl groups into organic molecules, motivated by the search for robust intermediates in pharmaceutical and agrochemical research. Labs across Europe began investigating sulfonyl chlorides, and with time, the focus turned to how a three-carbon chain—propane—could carry a sulfonyl chloride group. Interest grew as folks needed intermediates with stronger reactivity and better selectivity. Over the years, academic and industrial chemists sharpened up production routes, boosting safety and yield, and laid out the groundwork that many today take for granted during synthesis planning.
Product Overview
1-Propanesulfonyl Chloride does not stand out for charm. What it offers is a simple sulfonyl chloride moiety capped to the end of a straight propyl group. This makes it a handy tool for anyone who needs that touch of sulfur-based reactivity in a compact structure. Its directness gives it a straightforward role as an intermediate or building block—particularly for researchers working on sulfonamides, sulfonate esters, and various catalysts. In my own experience with small-molecule synthesis for pilot-scale pharmaceutical runs, sulfonyl chlorides like this one reduce steps and cut down on waste, especially compared to more cumbersome benzenesulfonyl variants.
Physical & Chemical Properties
Few things help chemists like a clear sense of what they are handling. This compound shows up as a colorless, strongly fuming liquid, with an expected sharp, acrid odor. Its molecular formula, C3H7ClO2S, unpacks to a molar mass around 144.61 g/mol. The boiling point usually lands between 163–168 °C, which means basic glassware covers most lab work. 1-Propanesulfonyl Chloride dissolves in common organic solvents—dichloromethane, ether, and even some alcohols—with a strong tendency to undergo hydrolysis in water, releasing HCl and corresponding sulfonic acids. Physiochemical data like flash point, vapor pressure, and specific gravity come in handy for anyone designing safe handling or scaling up—losing sight of these basics leads to more lab accidents than many admit.
Technical Specifications & Labeling
Manufacturers usually spell out the purity, most selling material at 98% or above unless the job calls for something purer. Impurities generally run to sulfonic acid or traces of dichloropropanes. Labels and safety data worksheets must list the UN number (UN 3265), hazard labels for corrosive and irritant properties, and the chemical abstract number (CAS 1132-12-9). My time in quality control taught me to never skip cross-checking these; labeling mistakes have a real cost, especially where transportation and customs rules get strict. Suppliers ought to provide batch analysis, shelf-life information, and remediation steps for accidental exposure or release.
Preparation Method
Preparation often follows a direct chlorination route. One-pot procedures mix 1-propanesulfonic acid with thionyl chloride or phosphorus pentachloride, generating HCl gas and the sulfonyl chloride. Much of the efficiency comes from keeping the system dry—moisture leads to decomposition and lost yield. Drying and purification need careful vacuum distillation or liquid-liquid extraction. In commercial plants, process intensification goes after continuous flow, tighter control of reaction exotherms, and better scrubber systems for off-gassing. Chemists with scale-up in mind often try to minimize chlorinated side products for easier downstream handling. The entire process stayed remarkably stable for decades, showing that simplicity and reliability never lose their charm.
Chemical Reactions & Modifications
1-Propanesulfonyl Chloride springs into action with nucleophiles—amines, alcohols, phenols, and sometimes even anionic carbon species. It rapidly forms sulfonamides and sulfonate esters; the sulfur–chlorine bond makes it eager for substitution. This knack helps in setting up more elaborate scaffolds for medicinal chemistry or polymer chemistry. Some labs, always after faster reactions or greener conditions, experiment with alternative bases or milder catalysts to tame the otherwise aggressive conditions. People working on catalyst design like to use propanesulfonyl groups for immobilizing ligands or modifying surfaces, which keeps the pace brisk in research and applications.
Synonyms & Product Names
Common naming conventions keep things practical. Besides 1-Propanesulfonyl Chloride, catalogs feature n-propanesulfonyl chloride, or even straight "propylsulfonyl chloride" in some circles. International suppliers might stretch to 1-chlorosulfonylpropane or Propane-1-sulfonyl chloride. It pays to cross-check synonyms to keep sourcing headaches to a minimum, as novel research often runs into language hiccups or translation errors.
Safety & Operational Standards
Folks handling this chemical need to suit up with goggles, gloves, and lab coats. It reacts strongly with moisture and can scorch tissue, especially skin and eyes. We ran safety drills for accidental splashes, since delayed response often causes lasting injuries. Adequate fume hoods and eye-wash stations become non-negotiable in every setting I have worked. Accidental inhalation, even at low doses, triggers cough, throat irritation, and occasionally longer-term effects on the respiratory tract. Storage demands tightly sealed, moisture-free bottles, ideally under an inert gas blanket for extended periods. Compliance with OSHA and local environmental requirements cannot be a checkbox exercise—record-keeping, waste disposal, and training audits prevent almost every major incident I have witnessed in industry.
Application Area
Work on pharmaceuticals, specialty chemicals, and agrochemicals often turns up a need for specific building blocks, and 1-Propanesulfonyl Chloride fits the bill. It forms sulfonamides that anchor some ACE inhibitors and anti-infectives, and it shows up in synthesis of cell-penetrating agents for delivery systems. Surface chemistry labs also prize propanesulfonyl groups for making water-compatible polymers or prepping surfaces for bioassays. Having a handy sulfonyl chloride with decent stability opens doors for new ion-exchange materials, lubricants, or even photoinitiators. Many chemists in scale-up or pilot plant roles lean into its versatility for crafting new scaffolds with reliable yields.
Research & Development
Research into alternatives to chlorinated solvents matters for labs aiming to trim environmental risk. Development teams hunt for methods that lower waste, boost yield, and cut costs from post-reaction purification. In past projects, we swapped slow, batchwise thionyl chloride additions with continuous microreactors, slashing downtime and improving disaster response time. Academic research homes in on less hazardous nucleophiles or greener conditions, benchmarking each tweak for effectiveness in downstream reactions. Some companies now diversify with stabilized or protected versions of propanesulfonyl intermediates, stretching the toolbox for medicinal and materials chemists.
Toxicity Research
Animal studies and cell culture models point to skin and respiratory tract corrosion as primary hazards. Inhalation or ingestion causes tissue necrosis, and repeated contact sometimes brings about chronic eczema or sensitization. I remember a colleague who underestimated the vapor hazard—repeated low-level exposures left them with persistent coughing for months. Regulatory bodies rate 1-Propanesulfonyl Chloride as moderate to high risk without proper personal protective equipment. It scores high for aquatic toxicity too, so effluent streams need careful scrubbing or neutralization before disposal. Larger-scale facilities often bring in independent toxicologists to keep exposure levels honest and ensure compliance with shifting international standards.
Future Prospects
As demand rises for customized surfactants, biologically active molecules, or functional polymers, the versatility of sulfonyl chlorides—especially propanesulfonyl variants—keeps growing. The future may lean on greener synthetic routes, aiming for catalysts that lower hazardous byproducts or processes that reclaim unreacted reagents on the fly. Many see potential in automated reaction monitoring or flow chemistry setups to bring finer control and speed to otherwise hazardous transformations. Advanced materials could draw on new ways to tether propanesulfonyl groups onto surfaces or nanoparticles, driving innovation in sensors and medical devices. Collaborative efforts between academic groups and industrial partners offer hope for increased recyclability, better safety, and smaller footprints across the chemical industry.
Digging into the Details
1-Propanesulfonyl chloride sounds technical, and to a lot of people, it probably feels like jargon from a chemistry textbook. Still, for anyone who spends time in labs—whether in research, pharmaceuticals, or specialty chemicals—knowing what sits behind such a name matters a lot. The chemical formula for 1-Propanesulfonyl chloride is C3H7SO2Cl. Not just a random collection of letters and numbers, this formula packs a story about molecular structure, use, and even safety considerations.
How the Formula Shapes Its Role
Look at the backbone of the formula—C3H7 hints at a three-carbon chain, a simple propyl group. SO2 shows up next, marking the sulfonyl functional group, and Cl rounds things off as the chloride. Anyone who’s worked with sulfonyl chlorides knows that this sort of arrangement leads straight to strong reactivity, especially toward nucleophiles like amines and alcohols. In my early days in a chemical synthesis lab, I found out the hard way why the presence of the chloride atom sets off alarms for moisture and gloves.
The chloride is reactive, jumping at any chance to bond with other molecules by swapping with another group. That makes 1-Propanesulfonyl chloride valuable as a building block in creating new compounds, including sulfonamides and other derivatives found in dyes, drugs, and agrochemicals. Most organic chemists keep compounds like this in their toolkit because reactions using sulfonyl chlorides help build stable linkages—not always obvious from reading the formula on paper, but clear when working on real synthesis where yield and purity make or break a project.
Safety and Handling—Not Just Paperwork
The Cl at the end of the formula signals more than just reactivity; it means that 1-Propanesulfonyl chloride needs respect in handling. The fumes cause respiratory irritation, and even short exposure can set off coughing fits or worse. I once witnessed a bench mate learn this lesson—one whiff, and he was out of the lab for the rest of the day. Never underestimate the safety goggles and fume hood, because the formula tells you what to expect, but not how strong the impact can be if you ignore best practices.
SDS sheets reinforce what hands-on experience teaches. Accidents tend to happen when the focus slips or training falls short. Good protocols, quick access to eyewash, and up-to-date training lower these risks substantially. Chemical safety culture grows stronger not through fear or rules, but through the combination of knowledge, habit, and respect for clean technique.
Why It Matters—Beyond Just a Name
1-Propanesulfonyl chloride stands for more than its formula. The formula traces back to thousands of experiments, commercial processes, and regulatory checks. A supply chain in the pharma industry relies on compounds like this one, as do researchers turning out new molecules for disease detection, polymer science, and environmental monitoring.
Solutions looking ahead could involve better material handling automation and greener alternatives that avoid using harsh chlorinated intermediates, reducing health risks and environmental impact. Research into less hazardous sulfonylating agents and improvements in engineering controls also promise measurable benefits. Education and investment in safer technology keep the risks manageable and the benefits available. Knowledge tied to these details lifts safety, productivity, and innovation across every sector that uses compounds like C3H7SO2Cl.
More Than Just a Chemical on a Shelf
1-Propanesulfonyl chloride doesn't catch many eyes outside chemical circles, yet its uses touch everyday products in ways most folks don't notice. In the world of organic synthesis, this sulfonating agent helps build more complex molecules. Scientists and manufacturers value it for its ability to introduce sulfonyl groups without much fuss, giving them a tool to shape the properties and functions of the molecules they’re after.
Pharmaceuticals: The Engine Room of Drug Development
Drug makers use 1-propanesulfonyl chloride to add sulfonyl groups during synthesis of active pharmaceutical ingredients. This reaction can change the solubility or biological activity of a compound, nudging it toward longer shelf life or better performance in the body. Without building blocks like this, the long march to effective treatments would get stuck on more costly and slower routes. There’s a direct line between good chemical reagents and the growth in options doctors have on their prescription pads.
Fine Chemicals and Agrochemicals: Field to Factory
In crop chemistry, inventors rely on 1-propanesulfonyl chloride to fine-tune new herbicides and pesticides. These products demand precise control over chemical structure, often so the active molecules only hit their target species and degrade safely afterward. Tweaking a molecule with a sulfonyl group sometimes turns it from ineffective to essential, helping feed a growing population while reducing leftover chemical footprints in the environment.
Performance Chemicals: Everyday Upgrades
Beyond drugs and fields, this compound helps shape surfactants and special coatings. Surfactants, the workhorses in detergents and cleaners, owe much of their grease-busting talent to fine-tuned molecular structures. By letting manufacturers swap in specific chemical groups, 1-propanesulfonyl chloride supports the creation of products that break up oils more cleanly or work in harsher conditions. Coatings for electronics or specialty plastics pull from the same toolkit, locking in unique physical properties for performance under pressure—like keeping circuits clear of moisture or plastics stable under sunlight.
The Safety Side
People working with 1-propanesulfonyl chloride come up against genuine challenges. Its reactivity means gloves, proper ventilation, and eye protection aren’t optional extras. Firms who take worker safety seriously invest in proper handling protocols, enclosed reactors, and real training, because even small leaks or splashes can have health consequences. In a world with growing attention to workplace safety, this practice points the way for safer chemical handling across industries.
Toward Cleaner Chemistry
Concerns over waste and hazardous byproducts in sulfonylation reactions drive researchers to redesign old processes. Green chemistry pushes for methods that use less energy, avoid harmful solvents, and recover as much raw material as possible. Projects now blend process engineering with chemistry know-how—instead of settling for routes that work, teams keep searching for ones that work cleaner. The chemical industry’s ongoing shift toward sustainability benefits from honest conversations about trade-offs and a focus on practical, workable solutions.
Final Thoughts on Industrial Relevance
From first-hand stories of product development labs to the backrooms of agrochemical plants, the impact of a strong, reliable building block can be enormous. 1-propanesulfonyl chloride plays its part behind the scenes—a reminder that improvements in daily life often begin with choices made far upstream, in glass flasks and steel tanks. Progress in this field relies on experience, open exchange of results, and a stubborn drive to make things safer and more sustainable for the long haul.
Knowing What You're Really Dealing With
If you spend any time around chemical labs or manufacturing plants, you get used to the smell of sharp, strong reagents. 1-Propanesulfonyl chloride is one of those chemicals you know by reputation—and not in a friendly way. It reacts fiercely with water and gets even more unruly at higher temperatures. Nobody wants a splash of this stuff on bare skin, or, worse, in their eyes. It makes sense to get realistic about storage and handling, not just for your own safety, but for everyone who works nearby.
Storing to Stop Trouble Before It Starts
This chemical doesn't keep well with casual storage habits. Any room that gets used for long-term storage of 1-Propanesulfonyl chloride needs solid ventilation. I’ve opened cabinets stocked with sensitive chemicals and felt the sting in my nose—a sign venting is either non-existent or someone’s been sloppy with lids. Good air exchange stops vapor buildup, which nobody wants to breathe.
A dry, cool environment gives the chemical the kind of stability people can trust. Humidity is an enemy here. 1-Propanesulfonyl chloride breaks down when it touches water, releasing acid fumes that mess up both equipment and lungs. Containers require a tight seal, ideally glass or high-grade plastic with zero cracks or sloppy closures. If you keep the reagent anywhere near acids, bases, or things like strong oxidizers, you’re gambling with cross-reactions. I’ve seen spill reports where two incompatible bottles got stored side by side, and eventually, some unlabeled runoff crept from one onto the other, triggering a pretty nasty release.
Personal and Environmental Safety
Getting personal protection right isn’t optional. Whenever I handle sulfonyl chlorides, I reach for chemical splash goggles, not just glasses. Nitrile gloves (double up if you’re paranoid), lab coats or long sleeves, and closed-toe shoes are just the start. Even if you’re careful, static shocks or simple absent-mindedness can put you at risk. A face shield lives on my bench now after a friend’s close call during a transfer. Accidental breathing of these vapors can irritate the respiratory tract pretty quickly.
Vent hoods play a huge role. Working in open air—or thinking a cracked window will do—just isn’t wise. Fume hoods suck away the nasties before they find your lungs. Keep containers small enough that a dropped bottle stays manageable. People sometimes decant larger orders into working containers, but only after clear labeling and checking caps for any residue that could make fingers burn.
Avoiding Bigger Problems Down the Line
Disposal demands respect. Dumping it down the drain is reckless and illegal for a reason. Spills or leaks should get neutralized with sand or a chemical spill kit designed for acid chlorides. I’ve seen new techs try wiping small spills with paper towels, only to discover the fumes bite right through and leave a rash. You do it right by absorbing spills, neutralizing them—usually with sodium bicarbonate in small quantities—and bagging for proper hazardous waste pickup.
OSHA and local policies stay strict for good reason. Annual training refreshers sound dull, but they make sure everyone knows emergency showers, eyewash stations, and escape routes. Trying to muscle through exposure with “just water” doesn’t work if the chemical gets behind goggles or soaks through gloves. Skin cleaning under running water for at least 15 minutes stands between you and real harm.
Simple Rules That Make a Difference
Carelessness shortens careers, especially with potent reagents like 1-Propanesulfonyl chloride. Accurate labels, tight seals, fresh gloves, working in a vented area, and disposing of waste safely aren’t just red tape—each habit makes work safer for everyone. You might not spot a mistake every day, but a single slip can undo weeks of good practice. Relying on experience and respecting the chemical never steered me wrong.
Breaking Down the Basics
1-Propanesulfonyl chloride might not grab much attention outside a laboratory, but the information tied to this compound, including its CAS number, carries a lot of weight for chemists and manufacturers. The CAS number for 1-Propanesulfonyl chloride is 1633-83-6. This string of numbers isn’t just a label; it serves as an anchor point in tracking chemical inventory, research, and safety protocols around the world.
Behind the CAS Number
Anyone who spent long hours navigating chemical databases knows how comforting it feels to find the right CAS number. It’s short for Chemical Abstracts Service number, a unique numerical identifier for chemical substances. Instead of juggling translations of systematic or trade names, anyone from a small startup lab to a global corporation uses the CAS number to avoid costly mix-ups.
Errors in chemical identification don’t just cause minor storms in a flask. I remember one of my own projects, where a supplier listed a batch with a wrong CAS number. We caught the error before the reaction started, but only after a tense morning of sorting through datasheets. Sticking to the right numbers means suppliers, buyers, and research teams stay on the same page, keeping safety and efficiency intact.
Stakes Rise in Industrial and Lab Settings
Tracking compounds correctly saves more than time—it defends against accidents and protects workers. 1-Propanesulfonyl chloride behaves as a reactive compound, often used in synthesis of pharmaceuticals or specialty chemicals. Without accurate identification, the risk of improper storage or mixing increases sharply. The 1633-83-6 tag gives emergency responders clear information for safety procedures if any incident takes place.
I learned from working with similar reagents that the right CAS number speeds up sourcing and minimizes dangerous ambiguity. Whether ordering from catalogs or checking regulatory compliance for transport and disposal, the single numerical code translates directly to chemical structure without room for confusion.
Supporting Safe and Efficient Research
In academic research and commercial R&D alike, precise chemical tracking forms a backbone for collaboration. Journals often require authors to cite the CAS number alongside chemical names to help other labs replicate results. This streamlines peer review and makes fact-checking straightforward. If someone investigating new applications for 1-Propanesulfonyl chloride references 1633-83-6, colleagues worldwide can cross-check results instantly. This brings a level of transparency that builds trust in published findings.
Improved Solutions for Chemical Management
Better tools exist now for chemical management, such as inventory tracking software and digital lab notebooks, which embed CAS numbers for every reagent entered. These systems flag mismatches and reduce human error. Investing in these solutions helps businesses avoid regulatory trouble and supports greener practices by accurately tracking hazardous materials in the cycle from purchase to disposal.
Looking at my own experience, I saw smaller labs struggle with outdated or incomplete records. Adopting a standard like the CAS registry has paid off by simplifying audits and pinning accountability to each step in the chemical life cycle.
Final Thoughts on Making Numbers Work Harder
In the end, remembering the CAS number 1633-83-6 for 1-Propanesulfonyl chloride means banking on a system built to keep science, industry, and safety measures running smoothly. Every digit delivers a little extra confidence to the person about to mix, move, or monitor a reaction.
Understanding What Happens with 1-Propanesulfonyl Chloride
Chemistry labs always come packed with substances that demand attention for the risks they bring. 1-Propanesulfonyl chloride falls under that list. Handling chemicals with reactive groups like this one, especially those ending with "chloride," often sends up a red flag even before checking the safety sheet. In my early days working with sulfonyl chlorides, the sharp, pungent smell alone reminded me to keep my wits about me. Eyes would start to itch, and—if someone got careless—skin could flush up red and painful.
Real Hazards: Not a Theoretical Concern
This chemical reacts fiercely with water and moisture, generating hydrochloric acid as a byproduct. A splash on the countertop, a sweaty glove, or even slightly humid air can trigger that reaction. Hydrochloric acid in vapor or liquid form burns the skin and eyes. Over time, inhaling those fumes led a few colleagues to headaches or raw throats. From published incident reports, even experienced workers underestimated how much vapor could spread from a small spill.
The agency facts back up this concern. According to the European Chemicals Agency, 1-propanesulfonyl chloride can cause severe burns to skin, irreparable eye injury, and problems with breathing if inhaled. If swallowed, it’s not just about an upset stomach—emergency medical care becomes a must. Chemical burns left untreated can lead to scars or even loss of eyesight. Risk increases when people grow too comfortable and take shortcuts, like skipping their gloves to "just measure a quick gram" or “working under the fume hood but not wearing eye protection.”
Why Safety Steps Matter Every Day
The best way to sidestep the pain is to take precautions seriously, each and every time. No matter the rush, lab coats, chemical splash goggles, and thick nitrile gloves come before cracking open the bottle. Working under a well-maintained fume hood works for both large and tiny volumes. I learned from one bad shift—splashed a droplet on my sleeve, got a mild burn, and ruined a lesson plan for my chem lab students. Keeping spill kits and eyewash stations tested and nearby means not needing to scramble during those crucial moments.
Clean-up forms another hotspot. A chemist in my network once mopped up a spill without realizing his sponge was just spreading hydrochloric acid further. Proper neutralizers and clean procedures—like plenty of dry absorbent, acids diluted and neutralized stepwise—keep everyone safer and save money on damage control.
Training and Common Sense Go Hand in Hand
Every training I attended hammered home the same lesson: fix small habits before they turn into big problems. Label bottles clearly, store them dry and tight-sealed, and double-check that fume hoods don’t have airflow issues. Ask around—the people who've spent the most time in synthetic labs often have stories about accidents that pushed everyone to refresh their skills. Keeping emergency numbers and protocols posted on the wall lets everybody act fast if something goes wrong.
Building a Safer Workplace Together
The goal isn't to create anxiety every time you see sulfonyl chloride. Instead, it’s about an honest respect for what these materials can do. Teams that talk regularly about safety, share examples, and encourage reporting even small incidents see fewer injuries. Data from the U.S. Occupational Safety and Health Administration show that workplaces where people feel empowered to speak up about unsafe behavior or missing protective gear clock in fewer chemical accident days.
Respect for 1-propanesulfonyl chloride starts with good habits and an open culture. Every person in the lab, from student to senior chemist, plays a part in keeping themselves and their coworkers healthy enough to keep showing up—and not spend their time filling out incident forms or sitting in the emergency room.